
| IGHG1 | |||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Aliases | IGHG1 , immunoglobulin heavy constant gamma 1 (G1m marker) | ||||||||||||||||||||||||||||||||||||||||||||||||||
| External IDs | OMIM: 147100 GeneCards: IGHG1 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikidata | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
Ig gamma-1 chain C region is a protein that in humans is encoded by the IGHG1 gene. [3]

| IGHG1 | |||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Aliases | IGHG1 , immunoglobulin heavy constant gamma 1 (G1m marker) | ||||||||||||||||||||||||||||||||||||||||||||||||||
| External IDs | OMIM: 147100 GeneCards: IGHG1 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikidata | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
Ig gamma-1 chain C region is a protein that in humans is encoded by the IGHG1 gene. [3]

An antibody (Ab), also known as an immunoglobulin (Ig), is a large, Y-shaped protein used by the immune system to identify and neutralize foreign objects such as pathogenic bacteria and viruses. The antibody recognizes a unique molecule of the pathogen, called an antigen. Each tip of the "Y" of an antibody contains a paratope that is specific for one particular epitope on an antigen, allowing these two structures to bind together with precision. Using this binding mechanism, an antibody can tag a microbe or an infected cell for attack by other parts of the immune system, or can neutralize it directly.

Protein A is a 42 kDa surface protein originally found in the cell wall of the bacteria Staphylococcus aureus. It is encoded by the spa gene and its regulation is controlled by DNA topology, cellular osmolarity, and a two-component system called ArlS-ArlR. It has found use in biochemical research because of its ability to bind immunoglobulins. It is composed of five homologous Ig-binding domains that fold into a three-helix bundle. Each domain is able to bind proteins from many mammalian species, most notably IgGs. It binds the heavy chain within the Fc region of most immunoglobulins and also within the Fab region in the case of the human VH3 family. Through these interactions in serum, where IgG molecules are bound in the wrong orientation, the bacteria disrupts opsonization and phagocytosis.

The word allotype comes from two Greek roots, allo meaning 'other or differing from the norm' and typos meaning 'mark'. In immunology, allotype is an immunoglobulin variation that can be found among antibody classes and is manifested by heterogeneity of immunoglobulins present in a single vertebrate species. The structure of immunoglobulin polypeptide chain is dictated and controlled by number of genes encoded in the germ line. However, these genes, as it was discovered by serologic and chemical methods, could be highly polymorphic. This polymorphism is subsequently projected to the overall amino acid structure of antibody chains. Polymorphic epitopes can be present on immunoglobulin constant regions on both heavy and light chains, differing between individuals or ethnic groups and in some cases may pose as immunogenic determinants. Exposure of individuals to a non-self allotype might elicit an anti- allotype response and became cause of problems for example in a patient after transfusion of blood or in a pregnant woman. However, it is important to mention that not all variations in immunoglobulin amino acid sequence pose as a determinant responsible for immune response. Some of these allotypic determinants may be present at places that are not well exposed and therefore can be hardly serologically discriminated. In other cases, variation in one isotype can be compensated by the presence of this determinant on another antibody isotype in one individual. This means that divergent allotype of heavy chain of IgG antibody may be balanced by presence of this allotype on heavy chain of for example IgA antibody and therefore is called isoallotypic variant. Especially large number of polymorphisms were discovered in IgG antibody subclasses. Which were practically used in forensic medicine and in paternity testing, before replaced by modern day DNA fingerprinting.
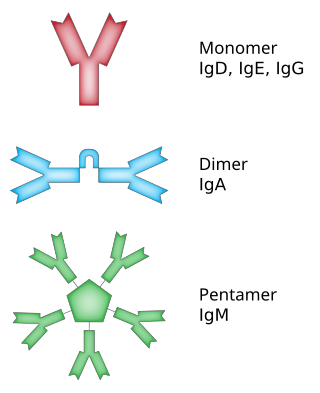
In immunology, antibodies are classified into several types called isotypes or classes. The variable (V) regions near the tip of the antibody can differ from molecule to molecule in countless ways, allowing it to specifically target an antigen . In contrast, the constant (C) regions only occur in a few variants, which define the antibody's class. Antibodies of different classes activate distinct effector mechanisms in response to an antigen . They appear at different stages of an immune response, differ in structural features, and in their location around the body.
Protein L was first isolated from the surface of bacterial species Peptostreptococcus magnus and was found to bind immunoglobulins through L chain interaction, from which the name was suggested. It consists of 719 amino acid residues. The molecular weight of protein L purified from the cell walls of Peptostreptoccus magnus was first estimated as 95kD by SDS-PAGE in the presence of reducing agent 2-mercaptoethanol, while the molecular weight was determined to 76kD by gel chromotography in the presence of 6 M guanidine HCl. Protein L does not contain any interchain disulfide loops, nor does it consist of disulfide-linked subunits. It is an acidic molecule with a pI of 4.0. Unlike protein A and protein G, which bind to the Fc region of immunoglobulins (antibodies), protein L binds antibodies through light chain interactions. Since no part of the heavy chain is involved in the binding interaction, Protein L binds a wider range of antibody classes than protein A or G. Protein L binds to representatives of all antibody classes, including IgG, IgM, IgA, IgE and IgD. Single chain variable fragments (scFv) and Fab fragments also bind to protein L.

The Joining (J) chain is a protein component that links monomers of antibodies IgM and IgA to form polymeric antibodies capable of secretion. The J chain is well conserved in the animal kingdom, but its specific functions are yet to be fully understood. It is a 137 residue polypeptide, encoded by the IGJ gene.

Polymeric immunoglobulin receptor (pIgR) is a transmembrane protein that in humans is encoded by the PIGR gene. It is an Fc receptor which facilitates the transcytosis of the soluble polymeric isoforms of immunoglobulin A and immunoglobulin M (pIg) and immune complexes. pIgRs are mainly located on the epithelial lining of mucosal surfaces of the gastrointestinal tract. The composition of the receptor is complex, including 6 immunoglobulin-like domains, a transmembrane region, and an intracellular domain. pIgR expression is under the strong regulation of cytokines, hormones, and pathogenic stimuli.

Low affinity immunoglobulin gamma Fc region receptor II-a is a protein that in humans is encoded by the FCGR2A gene.

Protein AMBP is a protein that in humans is encoded by the AMBP gene.
Cystatin-A is a protein that in humans is encoded by the CSTA gene.

Immunoglobulin kappa constant, also known as IGKC, is a human gene that encodes the constant domain of kappa-type light chains for antibodies. It is found on chromosome 2, in humans, within the Immunoglobulin kappa locus, IGK@.
Immunoglobulin lambda-like polypeptide 1 is a protein that in humans is encoded by the IGLL1 gene. IGLL1 has also recently been designated CD179B.

Ig mu chain C region is a protein that in humans is encoded by the IGHM gene.

Inter-alpha-trypsin inhibitor heavy chain H2 is a protein that in humans is encoded by the ITIH2 gene.

Immunoglobulin heavy constant alpha 1 is a immunoglobulin gene with symbol IGHA1. It encodes a constant (C) segment of Immunoglobulin A heavy chain. Immunoglobulin A is an antibody that plays a critical role in immune function in the mucous membranes. IgA shows the same typical structure of other antibody classes, with two heavy chains and two light chains, and four distinct domains: one variable region, and three variable regions. As a major class of immunoglobulin in body secretions, IgA plays a role in defending against infection, as well as preventing the access of foreign antigens to the immunologic system.

Cystatin-SA is a protein that in humans is encoded by the CST2 gene.

Ig gamma-2 chain C region is a protein that in humans is encoded by the IGHG2 gene.
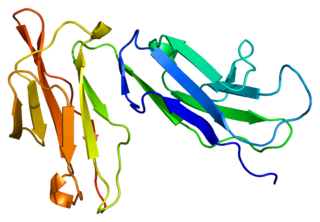
Fc fragment of IgG receptor IIb is a low affinity inhibitory receptor for the Fc region of immunoglobulin gamma (IgG). FCGR2B participates in the phagocytosis of immune complexes and in the regulation of antibody production by B lymphocytes.

Ig gamma-3 chain C region is a protein that in humans is encoded by the IGHG3 gene.

High affinity immunoglobulin epsilon receptor subunit beta is a protein that in humans is encoded by the MS4A2 gene.