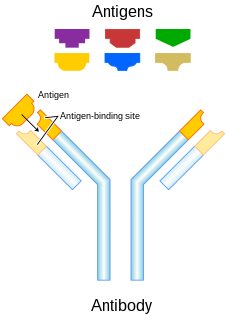
An antibody (Ab), also known as an immunoglobulin (Ig), is a large, Y-shaped protein used by the immune system to identify and neutralize foreign objects such as pathogenic bacteria and viruses. The antibody recognizes a unique molecule of the pathogen, called an antigen. Each tip of the "Y" of an antibody contains a paratope that is specific for one particular epitope on an antigen, allowing these two structures to bind together with precision. Using this binding mechanism, an antibody can tag a microbe or an infected cell for attack by other parts of the immune system, or can neutralize it directly.

The immunoglobulin heavy chain (IgH) is the large polypeptide subunit of an antibody (immunoglobulin). In human genome, the IgH gene loci are on chromosome 14.

The immunoglobulin light chain is the small polypeptide subunit of an antibody (immunoglobulin).

The word allotype comes from two Greek roots, allo meaning 'other or differing from the norm' and typos meaning 'mark'. In immunology, allotype is an immunoglobulin variation that can be found among antibody classes and is manifested by heterogeneity of immunoglobulins present in a single vertebrate species. The structure of immunoglobulin polypeptide chain is dictated and controlled by number of genes encoded in the germ line. However, these genes, as it was discovered by serologic and chemical methods, could be highly polymorphic. This polymorphism is subsequently projected to the overall amino acid structure of antibody chains. Polymorphic epitopes can be present on immunoglobulin constant regions on both heavy and light chains, differing between individuals or ethnic groups and in some cases may pose as immunogenic determinants. Exposure of individuals to a non-self allotype might elicit an anti- allotype response and became cause of problems for example in a patient after transfusion of blood or in a pregnant woman. However, it is important to mention that not all variations in immunoglobulin amino acid sequence pose as a determinant responsible for immune response. Some of these allotypic determinants may be present at places that are not well exposed and therefore can be hardly serologically discriminated. In other cases, variation in one isotype can be compensated by the presence of this determinant on another antibody isotype in one individual. This means that divergent allotype of heavy chain of IgG antibody may be balanced by presence of this allotype on heavy chain of for example IgA antibody and therefore is called isoallotypic variant. Especially large number of polymorphisms were discovered in IgG antibody subclasses. Which were practically used in forensic medicine and in paternity testing, before replaced by modern day DNA fingerprinting.

In immunology, antibodies are classified into several types called isotypes or classes. The variable (V) regions near the tip of the antibody can differ from molecule to molecule in countless ways, allowing it to specifically target an antigen . In contrast, the constant (C) regions only occur in a few variants, which define the antibody's class. Antibodies of different classes activate distinct effector mechanisms in response to an antigen . They appear at different stages of an immune response, differ in structural features, and in their location around the body.
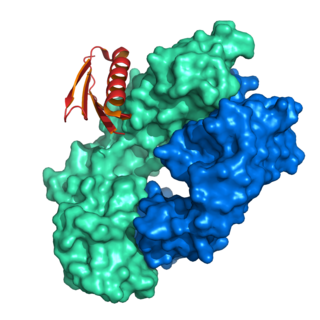
Protein L was first isolated from the surface of bacterial species Peptostreptococcus magnus and was found to bind immunoglobulins through L chain interaction, from which the name was suggested. It consists of 719 amino acid residues. The molecular weight of Protein L purified from the cell walls of Peptostreptoccus magnus was first estimated as 95kD by SDS-PAGE in the presence of reducing agent 2-mercaptoethanol, while the molecular weight was determined to 76kD by gel chromotography in the presence of 6 M guanidine HCl. Protein L does not contain any interchain disulfide loops, nor does it consist of disulfide-linked subunits. It is an acidic molecule with a pI of 4.0. Unlike Protein A and Protein G, which bind to the Fc region of immunoglobulins (antibodies), Protein L binds antibodies through light chain interactions. Since no part of the heavy chain is involved in the binding interaction, Protein L binds a wider range of antibody classes than Protein A or G. Protein L binds to representatives of all antibody classes, including IgG, IgM, IgA, IgE and IgD. Single chain variable fragments (scFv) and Fab fragments also bind to Protein L.

Cathepsin L1 is a protein that in humans is encoded by the CTSL1 gene. The protein is a cysteine cathepsin, a lysosomal cysteine protease that plays a major role in intracellular protein catabolism.

Protein-glutamine gamma-glutamyltransferase E is an enzyme that in humans is encoded by the TGM3 gene.

Lipoamide acyltransferase component of branched-chain alpha-keto acid dehydrogenase complex, mitochondrial is an enzyme that in humans is encoded by the DBT gene.

60S ribosomal protein L40 (RPL40) is a protein that in humans is encoded by the UBA52 gene.
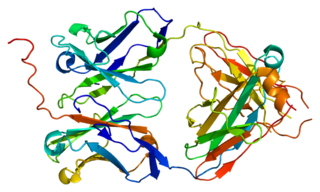
Immunoglobulin lambda-like polypeptide 1 is a protein that in humans is encoded by the IGLL1 gene. IGLL1 has also recently been designated CD179B.

Ig mu chain C region is a protein that in humans is encoded by the IGHM gene.

Ig gamma-1 chain C region is a protein that in humans is encoded by the IGHG1 gene.

Immunoglobulin heavy constant alpha 1 is a immunoglobulin gene with symbol IGHA1. It encodes a constant (C) segment of Immunoglobulin A heavy chain. Immunoglobulin A is an antibody that plays a critical role in immune function in the mucous membranes. IgA shows the same typical structure of other antibody classes, with two heavy chains and two light chains, and four distinct domains: one variable region, and three variable regions. As a major class of immunoglobulin in body secretions, IgA plays a role in defending against infection, as well as preventing the access of foreign antigens to the immunologic system.

Ig delta chain C region is a protein that in humans is encoded by the IGHD gene.
Immunoglobulin lambda locus, also known as IGL@, is a region on the q arm of human chromosome 22, region 11.22 (22q11.22) that contains genes for the lambda light chains of antibodies.

Sialic acid-binding Ig-like lectin 5 is a protein that in humans is encoded by the SIGLEC5 gene. SIGLEC5 has also been designated CD170.

Ig gamma-3 chain C region is a protein that in humans is encoded by the IGHG3 gene.
Immunoglobulin light chains that are circulating in serum in a free (unbound) state are called free light chains (FLCs). Measurement of the serum level of FLCs became practical as a clinical blood test in recent decades. These tests are used as an aid in the diagnosis and monitoring of multiple myeloma and related disorders. There are two types of immunoglobulin light chain produced in humans, designated by the Greek letters kappa (κ) and lambda (λ). Comparing the ratio of κ FLCs to λ FLCs in a person's serum against reference ranges indicates whether that person may have a plasma cell tumour such as multiple myeloma or AL amyloidosis.
The basic structure of immunoglobulin (Ig) molecules is a tetramer of two light chains and two heavy chains linked by disulphide bonds. There are two types of light chains: kappa and lambda, each composed of a constant domain (CL) and a variable domain (VL). There are five types of heavy chains: alpha, delta, epsilon, gamma and mu, all consisting of a variable domain (VH) and three or four constant domains. Ig molecules are highly modular proteins, in which the variable and constant domains have clear, conserved sequence patterns. The domains in Ig and Ig-like molecules are grouped into four types: V-set, C1-set, C2-set and I-set. Structural studies have shown that these domains share a common core Greek-key beta-sandwich structure, with the types differing in the number of strands in the beta-sheets as well as in their sequence patterns.














