
A nucleosome is the basic structural unit of DNA packaging in eukaryotes. The structure of a nucleosome consists of a segment of DNA wound around eight histone proteins and resembles thread wrapped around a spool. The nucleosome is the fundamental subunit of chromatin. Each nucleosome is composed of a little less than two turns of DNA wrapped around a set of eight proteins called histones, which are known as a histone octamer. Each histone octamer is composed of two copies each of the histone proteins H2A, H2B, H3, and H4.
RSC is a member of the ATP-dependent chromatin remodeler family. The activity of the RSC complex allows for chromatin to be remodeled by altering the structure of the nucleosome.

In molecular biology, SWI/SNF, is a subfamily of ATP-dependent chromatin remodeling complexes, which is found in eukaryotes. In other words, it is a group of proteins that associate to remodel the way DNA is packaged. This complex is composed of several proteins – products of the SWI and SNF genes, as well as other polypeptides. It possesses a DNA-stimulated ATPase activity that can destabilize histone-DNA interactions in reconstituted nucleosomes in an ATP-dependent manner, though the exact nature of this structural change is unknown. The SWI/SNF subfamily provides crucial nucleosome rearrangement, which is seen as ejection and/or sliding. The movement of nucleosomes provides easier access to the chromatin, allowing genes to be activated or repressed.
Chromatin remodeling is the dynamic modification of chromatin architecture to allow access of condensed genomic DNA to the regulatory transcription machinery proteins, and thereby control gene expression. Such remodeling is principally carried out by 1) covalent histone modifications by specific enzymes, e.g., histone acetyltransferases (HATs), deacetylases, methyltransferases, and kinases, and 2) ATP-dependent chromatin remodeling complexes which either move, eject or restructure nucleosomes. Besides actively regulating gene expression, dynamic remodeling of chromatin imparts an epigenetic regulatory role in several key biological processes, egg cells DNA replication and repair; apoptosis; chromosome segregation as well as development and pluripotency. Aberrations in chromatin remodeling proteins are found to be associated with human diseases, including cancer. Targeting chromatin remodeling pathways is currently evolving as a major therapeutic strategy in the treatment of several cancers.
Transcription activator BRG1 also known as ATP-dependent chromatin remodeler SMARCA4 is a protein that in humans is encoded by the SMARCA4 gene.

Probable global transcription activator SNF2L2 is a protein that in humans is encoded by the SMARCA2 gene.

SWI/SNF-related matrix-associated actin-dependent regulator of chromatin subfamily A member 5 is a protein that in humans is encoded by the SMARCA5 gene.

Actin-like protein 6A is a protein that in humans is encoded by the ACTL6A gene.

AT-rich interactive domain-containing protein 1A is a protein that in humans is encoded by the ARID1A gene.

SWI/SNF-related matrix-associated actin-dependent regulator of chromatin subfamily E member 1 is a protein that in humans is encoded by the SMARCE1 gene.

SWI/SNF-related matrix-associated actin-dependent regulator of chromatin subfamily D member 1 is a protein that in humans is encoded by the SMARCD1 gene.

SWI/SNF-related matrix-associated actin-dependent regulator of chromatin subfamily D member 3 is a protein that in humans is encoded by the SMARCD3 gene.

Probable global transcription activator SNF2L1 is a protein that in humans is encoded by the SMARCA1 gene.
In the field of molecular biology, the Mi-2/NuRDcomplex, is a group of associated proteins with both ATP-dependent chromatin remodeling and histone deacetylase activities. As of 2007, Mi-2/NuRD was the only known protein complex that couples chromatin remodeling ATPase and chromatin deacetylation enzymatic functions.
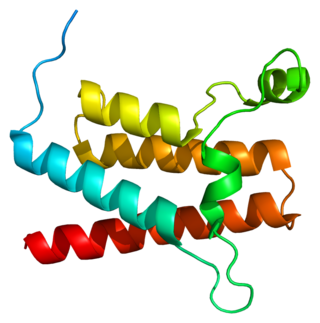
In molecular biology, the HAND domain is a protein domain which adopts a secondary structure consisting of four alpha helices, three of which form an L-like configuration. Helix H2 runs antiparallel to helices H3 and H4, packing closely against helix H4, whilst helix H1 reposes in the concave surface formed by these three helices and runs perpendicular to them. This domain confers DNA and nucleosome binding properties to the proteins in which it occurs. It is named the HAND domain because its 4-helical structure resembles an open hand.
In molecular biology, the WAC domain is a protein domain found on the N-terminus of WSTF protein. Its function is still unknown, but putatively thought to be involved in cell growth. The protein domain has been found to be present in both prokaryotes and eukaryotes
Nucleosome Remodeling Factor (NURF) is an ATP-dependent chromatin remodeling complex first discovered in Drosophila melanogaster that catalyzes nucleosome sliding in order to regulate gene transcription. It contains an ISWI ATPase, making it part of the ISWI family of chromatin remodeling complexes. NURF is highly conserved among eukaryotes and is involved in transcriptional regulation of developmental genes.
The INO80 subfamily of chromatin remodeling complexes are ATPases, and includes the INO80 and SWR1 complexes.
Chromodomain helicase DNA-binding (CHD) proteins is a subfamily of ATP-dependent chromatin remodeling complexes (remodelers). All remodelers fall under the umbrella of RNA/DNA helicase superfamily 2. In yeast, CHD complexes are primarily responsible for nucleosome assembly and organization. These complexes play an additional role in multicellular eukaryotes, assisting in chromatin access and nucleosome editing.
Robert E. Kingston is an American biochemist who studies the functional and regulatory role nucleosomes play in gene expression, specifically during early development. After receiving his PhD (1981) and completing post-doctoral research, Kingston became an assistant professor at Massachusetts General Hospital (1985), where he started a research laboratory focused on understanding chromatin's structure with regards to transcriptional regulation. As a Harvard graduate himself, Kingston has served his alma mater through his leadership.











