
In immunology, an antigen (Ag) is a molecule, moiety, foreign particulate matter, or an allergen, such as pollen, that can bind to a specific antibody or T-cell receptor. The presence of antigens in the body may trigger an immune response.

A cytotoxic T cell (also known as TC, cytotoxic T lymphocyte, CTL, T-killer cell, cytolytic T cell, CD8+ T-cell or killer T cell) is a T lymphocyte (a type of white blood cell) that kills cancer cells, cells that are infected by intracellular pathogens (such as viruses or bacteria), or cells that are damaged in other ways.

The T helper cells (Th cells), also known as CD4+ cells or CD4-positive cells, are a type of T cell that play an important role in the adaptive immune system. They aid the activity of other immune cells by releasing cytokines. They are considered essential in B cell antibody class switching, breaking cross-tolerance in dendritic cells, in the activation and growth of cytotoxic T cells, and in maximizing bactericidal activity of phagocytes such as macrophages and neutrophils. CD4+ cells are mature Th cells that express the surface protein CD4. Genetic variation in regulatory elements expressed by CD4+ cells determines susceptibility to a broad class of autoimmune diseases.

The major histocompatibility complex (MHC) is a large locus on vertebrate DNA containing a set of closely linked polymorphic genes that code for cell surface proteins essential for the adaptive immune system. These cell surface proteins are called MHC molecules.

Natural killer cells, also known as NK cells, are a type of cytotoxic lymphocyte critical to the innate immune system. They are a kind of large granular lymphocytes (LGL), and belong to the rapidly expanding family of known innate lymphoid cells (ILC) and represent 5–20% of all circulating lymphocytes in humans. The role of NK cells is analogous to that of cytotoxic T cells in the vertebrate adaptive immune response. NK cells provide rapid responses to virus-infected cells, stressed cells, tumor cells, and other intracellular pathogens based on signals from several activating and inhibitory receptors. Most immune cells detect the antigen presented on major histocompatibility complex I (MHC-I) on infected cell surfaces, but NK cells can recognize and kill stressed cells in the absence of antibodies and MHC, allowing for a much faster immune reaction. They were named "natural killers" because of the notion that they do not require activation to kill cells that are missing "self" markers of MHC class I. This role is especially important because harmful cells that are missing MHC I markers cannot be detected and destroyed by other immune cells, such as T lymphocyte cells.
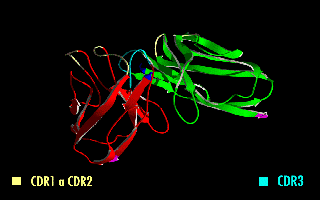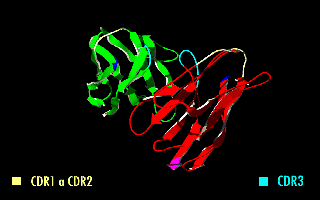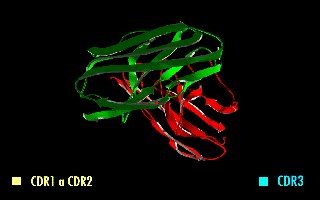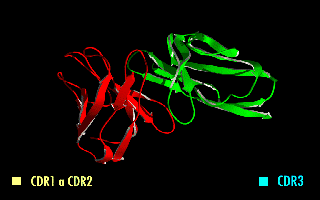
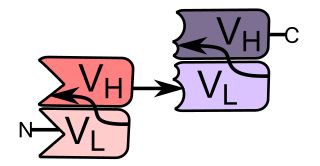
A single-chain variable fragment (scFv) is not actually a fragment of an antibody, but instead is a fusion protein of the variable regions of the heavy (VH) and light chains (VL) of immunoglobulins, connected with a short linker peptide of ten to about 25 amino acids. The linker is usually rich in glycine for flexibility, as well as serine or threonine for solubility, and can either connect the N-terminus of the VH with the C-terminus of the VL, or vice versa. This protein retains the specificity of the original immunoglobulin, despite removal of the constant regions and the introduction of the linker. The image to the right shows how this modification usually leaves the specificity unaltered.
Antigen processing, or the cytosolic pathway, is an immunological process that prepares antigens for presentation to special cells of the immune system called T lymphocytes. It is considered to be a stage of antigen presentation pathways. This process involves two distinct pathways for processing of antigens from an organism's own (self) proteins or intracellular pathogens, or from phagocytosed pathogens ; subsequent presentation of these antigens on class I or class II major histocompatibility complex (MHC) molecules is dependent on which pathway is used. Both MHC class I and II are required to bind antigens before they are stably expressed on a cell surface. MHC I antigen presentation typically involves the endogenous pathway of antigen processing, and MHC II antigen presentation involves the exogenous pathway of antigen processing. Cross-presentation involves parts of the exogenous and the endogenous pathways but ultimately involves the latter portion of the endogenous pathway.

An antigen-presenting cell (APC) or accessory cell is a cell that displays an antigen bound by major histocompatibility complex (MHC) proteins on its surface; this process is known as antigen presentation. T cells may recognize these complexes using their T cell receptors (TCRs). APCs process antigens and present them to T cells.

The T-cell receptor (TCR) is a protein complex found on the surface of T cells, or T lymphocytes, that is responsible for recognizing fragments of antigen as peptides bound to major histocompatibility complex (MHC) molecules. The binding between TCR and antigen peptides is of relatively low affinity and is degenerate: that is, many TCRs recognize the same antigen peptide and many antigen peptides are recognized by the same TCR.
CD8 is a transmembrane glycoprotein that serves as a co-receptor for the T-cell receptor (TCR). Along with the TCR, the CD8 co-receptor plays a role in T cell signaling and aiding with cytotoxic T cell-antigen interactions.

CD3 is a protein complex and T cell co-receptor that is involved in activating both the cytotoxic T cell and T helper cells. It is composed of four distinct chains. In mammals, the complex contains a CD3γ chain, a CD3δ chain, and two CD3ε chains. These chains associate with the T-cell receptor (TCR) and the CD3-zeta (ζ-chain) to generate an activation signal in T lymphocytes. The TCR, CD3-zeta, and the other CD3 molecules together constitute the TCR complex.

Minor histocompatibility antigen are peptides presented on the cellular surface of donated organs that are known to give an immunological response in some organ transplants. They cause problems of rejection less frequently than those of the major histocompatibility complex (MHC). Minor histocompatibility antigens (MiHAs) are diverse, short segments of proteins and are referred to as peptides. These peptides are normally around 9-12 amino acids in length and are bound to both the major histocompatibility complex (MHC) class I and class II proteins. Peptide sequences can differ among individuals and these differences arise from SNPs in the coding region of genes, gene deletions, frameshift mutations, or insertions. About a third of the characterized MiHAs come from the Y chromosome. Prior to becoming a short peptide sequence, the proteins expressed by these polymorphic or diverse genes need to be digested in the proteasome into shorter peptides. These endogenous or self peptides are then transported into the endoplasmic reticulum with a peptide transporter pump called TAP where they encounter and bind to the MHC class I molecule. This contrasts with MHC class II molecules's antigens which are peptides derived from phagocytosis/endocytosis and molecular degradation of non-self entities' proteins, usually by antigen-presenting cells. MiHA antigens are either ubiquitously expressed in most tissue like skin and intestines or restrictively expressed in the immune cells.
A bispecific monoclonal antibody is an artificial protein that can simultaneously bind to two different types of antigen or two different epitopes on the same antigen. Naturally occurring antibodies typically only target one antigen. BsAbs can be manufactured in several structural formats. BsAbs can be designed to recruit and activate immune cells, to interfere with receptor signaling and inactivate signaling ligands, and to force association of protein complexes. BsAbs have been explored for cancer immunotherapy, drug delivery, and Alzheimer's disease.
Bi-specific T-cell engagers (BiTEs) are a class of artificial bispecific monoclonal antibodies that are investigated for use as anti-cancer drugs. They direct a host's immune system, more specifically the T cells' cytotoxic activity, against cancer cells. BiTE is a registered trademark of Micromet AG.
Short Course Immune Induction Therapy or SCIIT, is a therapeutic strategy employing rapid, specific, short term-modulation of the immune system using a therapeutic agent to induce T-cell non-responsiveness, also known as operational tolerance. As an alternative strategy to immunosuppression and antigen-specific tolerance inducing therapies, the primary goal of SCIIT is to re-establish or induce peripheral immune tolerance in the context of autoimmune disease and transplant rejection through the use of biological agents. In recent years, SCIIT has received increasing attention in clinical and research settings as an alternative to immunosuppressive drugs currently used in the clinic, drugs which put the patients at risk of developing infection, cancer, and cardiovascular disease.
Kinetic-segregation is a model proposed for the mechanism of T-cell receptor (TCR) triggering. It offers an explanation for how TCR binding to its ligand triggers T-cell activation, based on size-sensitivity for the molecules involved. Simon J. Davis and Anton van der Merwe, University of Oxford, proposed this model in 1996. According to the model, TCR signalling is initiated by segregation of phosphatases with large extracellular domains from the TCR complex when binding to its ligand, allowing small kinases to phosphorylate intracellular domains of the TCR without inhibition. Its might also be applicable to other receptors of the Non-catalytic tyrosine-phosphorylated receptors family such as CD28.

Immunocore is a global commercial-stage biotechnology company, based in Oxfordshire, which researches and develops biological drugs using soluble T-cell receptor technology.
Immunodominance is the immunological phenomenon in which immune responses are mounted against only a few of the antigenic peptides out of the many produced. That is, despite multiple allelic variations of MHC molecules and multiple peptides presented on antigen presenting cells, the immune response is skewed to only specific combinations of the two. Immunodominance is evident for both antibody-mediated immunity and cell-mediated immunity. Epitopes that are not targeted or targeted to a lower degree during an immune response are known as subdominant epitopes. The impact of immunodominance is immunodomination, where immunodominant epitopes will curtail immune responses against non-dominant epitopes. Antigen-presenting cells such as dendritic cells, can have up to six different types of MHC molecules for antigen presentation. There is a potential for generation of hundreds to thousands of different peptides from the proteins of pathogens. Yet, the effector cell population that is reactive against the pathogen is dominated by cells that recognize only a certain class of MHC bound to only certain pathogen-derived peptides presented by that MHC class. Antigens from a particular pathogen can be of variable immunogenicity, with the antigen that stimulates the strongest response being the immunodominant one. The different levels of immunogenicity amongst antigens forms what is known as dominance hierarchy.
The p-i concept refers to the pharmacological interaction of drugs with immune receptors. It explains a form of drug hypersensitivity, namely T cell stimulation, which can lead to various acute inflammatory manifestations such as exanthems, eosinophilia and systemic symptoms, Stevens–Johnson syndrome, toxic epidermal nercrolysis, and complications upon withdrawing the drug.
Bahija Jallal is chief executive officer and director of the board of Immunocore. She has previously been president at MedImmune and AstraZeneca. She is a council member of the Government–University–Industry Research Roundtable of the National Academies of Sciences, Engineering and Medicine.











