Related Research Articles

An artificial cardiac pacemaker, commonly referred to as simply a pacemaker, is an implanted medical device that generates electrical pulses delivered by electrodes to one or more of the chambers of the heart. Each pulse causes the targeted chamber(s) to contract and pump blood, thus regulating the function of the electrical conduction system of the heart.
A dentist, also known as a dental surgeon, is a health care professional who specializes in dentistry, the branch of medicine focused on the teeth, gums, and mouth. The dentist's supporting team aids in providing oral health services. The dental team includes dental assistants, dental hygienists, dental technicians, and sometimes dental therapists.

An implantable cardioverter-defibrillator (ICD) or automated implantable cardioverter defibrillator (AICD) is a device implantable inside the body, able to perform defibrillation, and depending on the type, cardioversion and pacing of the heart. The ICD is the first-line treatment and prophylactic therapy for patients at risk for sudden cardiac death due to ventricular fibrillation and ventricular tachycardia.

A dental implant is a prosthesis that interfaces with the bone of the jaw or skull to support a dental prosthesis such as a crown, bridge, denture, or facial prosthesis or to act as an orthodontic anchor. The basis for modern dental implants is a biological process called osseointegration, in which materials such as titanium or zirconia form an intimate bond to the bone. The implant fixture is first placed so that it is likely to osseointegrate, then a dental prosthetic is added. A variable amount of healing time is required for osseointegration before either the dental prosthetic is attached to the implant or an abutment is placed which will hold a dental prosthetic or crown.

An implant is a medical device manufactured to replace a missing biological structure, support a damaged biological structure, or enhance an existing biological structure. For example, an implant may be a rod, used to strengthen weak bones. Medical implants are human-made devices, in contrast to a transplant, which is a transplanted biomedical tissue. The surface of implants that contact the body might be made of a biomedical material such as titanium, silicone, or apatite depending on what is the most functional. In 2018, for example, American Elements developed a nickel alloy powder for 3D printing robust, long-lasting, and biocompatible medical implants. In some cases implants contain electronics, e.g. artificial pacemaker and cochlear implants. Some implants are bioactive, such as subcutaneous drug delivery devices in the form of implantable pills or drug-eluting stents.

Streptococcus mutans is a facultatively anaerobic, gram-positive coccus commonly found in the human oral cavity and is a significant contributor to tooth decay. It is part of the "streptococci", an informal general name for all species in the genus Streptococcus. The microbe was first described by James Kilian Clarke in 1924.
Periodontology or periodontics is the specialty of dentistry that studies supporting structures of teeth, as well as diseases and conditions that affect them. The supporting tissues are known as the periodontium, which includes the gingiva (gums), alveolar bone, cementum, and the periodontal ligament. A periodontist is a dentist that specializes in the prevention, diagnosis and treatment of periodontal disease and in the placement of dental implants.
Dental plaque is a biofilm of microorganisms that grows on surfaces within the mouth. It is a sticky colorless deposit at first, but when it forms tartar, it is often brown or pale yellow. It is commonly found between the teeth, on the front of teeth, behind teeth, on chewing surfaces, along the gumline (supragingival), or below the gumline cervical margins (subgingival). Dental plaque is also known as microbial plaque, oral biofilm, dental biofilm, dental plaque biofilm or bacterial plaque biofilm. Bacterial plaque is one of the major causes for dental decay and gum disease.

A ventricular assist device (VAD) is an electromechanical device that provides support for cardiac pump function, which is used either to partially or to completely replace the function of a failing heart. VADs can be used in patients with acute or chronic heart failure, which can occur due to coronary artery disease, atrial fibrillation, valvular disease, and other conditions.

Maxillary sinus floor augmentation is a surgical procedure which aims to increase the amount of bone in the posterior maxilla, in the area of the premolar and molar teeth, by lifting the lower Schneiderian membrane and placing a bone graft.
Oral and maxillofacial pathology refers to the diseases of the mouth, jaws and related structures such as salivary glands, temporomandibular joints, facial muscles and perioral skin. The mouth is an important organ with many different functions. It is also prone to a variety of medical and dental disorders.
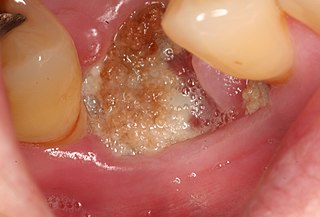
Medication-related osteonecrosis of the jaw is progressive death of the jawbone in a person exposed to a medication known to increase the risk of disease, in the absence of a previous radiation treatment. It may lead to surgical complication in the form of impaired wound healing following oral and maxillofacial surgery, periodontal surgery, or endodontic therapy.

Gingivitis is a non-destructive disease that causes inflammation of the gums; ulitis is an alternative term. The most common form of gingivitis, and the most common form of periodontal disease overall, is in response to bacterial biofilms that are attached to tooth surfaces, termed plaque-induced gingivitis. Most forms of gingivitis are plaque-induced.
Pacemaker failure is the inability of an implanted artificial pacemaker to perform its intended function of regulating the beating of the heart. A pacemaker uses electrical impulses delivered by electrodes in order to contract the heart muscles. Failure of a pacemaker is defined by the requirement of repeat surgical pacemaker-related procedures after the initial implantation. Most implanted pacemakers are dual chambered and have two leads, causing the implantation time to take longer because of this more complicated pacemaker system. These factors can contribute to an increased rate of complications which can lead to pacemaker failure.

Peri-implantitis is a destructive inflammatory process affecting the soft and hard tissues surrounding dental implants. The soft tissues become inflamed whereas the alveolar bone, which surrounds the implant for the purposes of retention, is lost over time.
Metallosis is the medical condition involving deposition and build-up of metal debris in the soft tissues of the body.
Peri-implant mucositis is defined as an inflammatory lesion of the peri-implant mucosa in the absence of continuing marginal bone loss.
Attempts in the last decade to develop surgical treatments based on MRI and CAT scans now receive less attention. These techniques are reserved for the most difficult cases where other therapeutic modalities have failed. The American Society of Maxillofacial Surgeons recommends a conservative/non-surgical approach first. Only 20% of patients need to proceed to surgery.
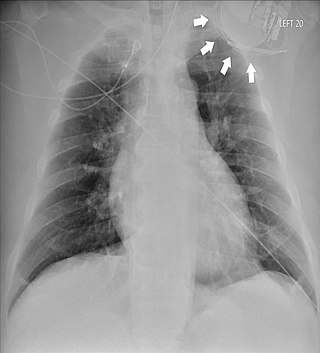
Twiddler's syndrome is a malfunction of a pacemaker due to manipulation of the device and the consequent dislodging of the leads from their intended location. As the leads move, they stop pacing the heart and can cause strange symptoms such as phrenic nerve stimulation resulting in abdominal pulsing or brachial plexus stimulation resulting in rhythmic arm twitching. Twiddler's syndrome in patients with an implanted defibrilator may lead to inadequate, painful defibrillation-shocks.
A root-analogue dental implant (RAI) – also known as a truly anatomic dental implant, or an anatomical/custom implant – is a medical device to replace one or more roots of a single tooth immediately after extraction. In contrast to common titanium screw type implants, these implants are custom-made to exactly match the extraction socket of the specific patient. Thus there is usually no need for surgery.
References
- ↑ Meier, Barry (22 January 2013). "Maker Aware of 40% Failure in Hip Implant - NYTimes.com". The New York Times . New York. ISSN 0362-4331 . Retrieved 5 June 2013.
- ↑ Balkany TJ; Hodges AV; Buchman CA; Luxford WM; Pillsbury CH; Roland PS; Shallop JK; Backous DD; Franz D; Graham JM; Hirsch B; Luntz M; Niparko JK; Patrick J; Payne SL; Telischi FF; Tobey EA; Truy E; Staller S (2005). "Cochlear implant soft failures consensus development conference statement". Otol. Neurotol. 26 (4): 815–8. doi:10.1097/01.mao.0000178150.44505.52. PMID 16015190. S2CID 23950969.
- ↑ "Association for the Advancement of Medical Instrumentation : CI - Cochlear Implants Committee". standards.aami.org. Archived from the original on 2014-07-12.
- ↑ Galagali, Girish; Reddy, E. Srinivas; Nidawani, Prakash; S P Behera, Sidhartha; Preetham, Pavan; Sarpangala, Mythri (January–March 2014). "Implant Failures: A Comprehensive Review" (PDF). International Journal of Preventive & Clinical Dental Research. 2014, 1(1): 11–17.
- ↑ Tissue-integrated prostheses :osseointegration in clinical dentistry, Per-Ingavar Branemark, George A. Zarb, Tomas Albrektsson, 1985
- ↑ Moy, P. K.; Medina, D.; Shetty, V.; Aghaloo, T. L. (2005). "Dental implant failure rates and associated risk factors". The International Journal of Oral & Maxillofacial Implants. 20 (4): 569–577. PMID 16161741.
- ↑ De Bruyn, H.; Collaert, B. (1994). "The effect of smoking on early implant failure". Clinical Oral Implants Research. 5 (4): 260–264. doi:10.1034/j.1600-0501.1994.050410.x. PMID 7640341.
- ↑ Watt, Holly; Newell, Claire (24 Oct 2012). "Faulty medical implants investigation: Patients failed by poor implant regulation, say surgeons - Telegraph". The Daily Telegraph . London. ISSN 0307-1235. OCLC 49632006. Archived from the original on 25 October 2012. Retrieved 5 June 2013.
- ↑ Zuckerman, Diana (2011). "Medical Device Recalls and the FDA Approval Process". Archives of Internal Medicine. 171 (11): 1006–11. doi: 10.1001/archinternmed.2011.30 . PMID 21321283.