
In immunology, an antigen (Ag) is any molecule, molecular structure, foreign particulate matter, or pollen grain that can bind to a specific antibody or T-cell receptor. The presence of antigens in the body may trigger an immune response. Antigens can be proteins, peptides, polysaccharides, lipids, or nucleic acids.
Immunotherapy or biological therapy is the treatment of disease by activating or suppressing the immune system. Immunotherapies designed to elicit or amplify an immune response are classified as activation immunotherapies, while immunotherapies that reduce or suppress are classified as suppression immunotherapies. Immunotherapy is under preliminary research for its potential to treat various forms of cancer.
A cancer vaccine is a vaccine that either treats existing cancer or prevents development of cancer. Vaccines that treat existing cancer are known as therapeutic cancer vaccines or tumor antigen vaccines. Some of the vaccines are "autologous", being prepared from samples taken from the patient, and are specific to that patient.

Cancer immunotherapy is the stimulation of the immune system to treat cancer, improving on the immune system's natural ability to fight the disease. It is an application of the fundamental research of cancer immunology and a growing subspecialty of oncology.
An oncolytic virus is a virus that preferentially infects and kills cancer cells. As the infected cancer cells are destroyed by oncolysis, they release new infectious virus particles or virions to help destroy the remaining tumour. Oncolytic viruses are thought not only to cause direct destruction of the tumour cells, but also to stimulate host anti-tumour immune system responses. Oncolytic viruses also have the ability to affect the tumor micro-environment in multiples ways.
Virotherapy is a treatment using biotechnology to convert viruses into therapeutic agents by reprogramming viruses to treat diseases. There are three main branches of virotherapy: anti-cancer oncolytic viruses, viral vectors for gene therapy and viral immunotherapy. These branches use three different types of treatment methods: gene overexpression, gene knockout, and suicide gene delivery. Gene overexpression adds genetic sequences that compensate for low to zero levels of needed gene expression. Gene knockout uses RNA methods to silence or reduce expression of disease-causing genes. Suicide gene delivery introduces genetic sequences that induce an apoptotic response in cells, usually to kill cancerous growths. In a slightly different context, virotherapy can also refer more broadly to the use of viruses to treat certain medical conditions by killing pathogens.
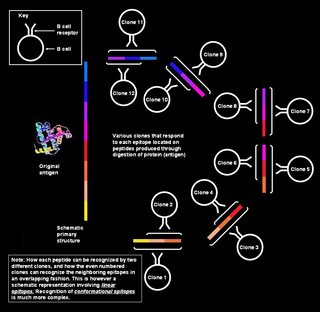
Polyclonal B cell response is a natural mode of immune response exhibited by the adaptive immune system of mammals. It ensures that a single antigen is recognized and attacked through its overlapping parts, called epitopes, by multiple clones of B cell.
An oncoantigen is a surface or soluble tumor antigen that supports tumor growth. A major problem of cancer immunotherapy is the selection of tumor cell variants that escape immune recognition. The notion of oncoantigen was set forth in the context of cancer immunoprevention to define a class of persistent tumor antigens not prone to escape from immune recognition.
Peptide-based synthetic vaccines, also called epitope vaccines, are subunit vaccines made from peptides. The peptides mimic the epitopes of the antigen that triggers direct or potent immune responses. Peptide vaccines can not only induce protection against infectious pathogens and non-infectious diseases but also be utilized as therapeutic cancer vaccines, where peptides from tumor-associated antigens are used to induce an effective anti-tumor T-cell response.
JX-594 is an oncolytic virus is designed to target and destroy cancer cells. It is also known as Pexa-Vec, INN pexastimogene devacirepvec) and was constructed in Dr. Edmund Lattime's lab at Thomas Jefferson University, tested in clinical trials on melanoma patients, and licensed and further developed by SillaJen.
Ursula Wiedermann is an Austrian medical scientist who has made significant contributions in the field of allergies and of cancer immunotherapy. She is currently Professor of Vaccinology at the Medical University of Vienna. Wiedermann's work in the field of B cell peptide vaccines led to the creation of HER-Vaxx, an immunotherapy for the treatment of HER-2-positive cancers. This vaccine is currently being taken into mid-stage clinical development in gastric cancer by the biotech company Imugene, where Wiedermann is Chief Scientific Officer.
Viralytics Ltd is an Australian biotechnology company working in the field of oncolytic viruses, that is, viruses that preferentially infect and kill cancer cells. The company's oncolytic virus product, called Cavatak, is currently in clinical trials in metastatic melanoma and other cancers. The drug was granted Orphan Drug status in advanced melanoma in December 2005.
Prescient Therapeutics Ltd is a clinical stage oncology company. The company is focused on the development of a universal CAR-T platform (OmniCAR), enhanced CAR-T cell manufacturing & function (CellPryme) and on two small molecule drug targeted therapies.

PD-1 inhibitors and PD-L1 inhibitors are a group of checkpoint inhibitor anticancer drugs that block the activity of PD-1 and PDL1 immune checkpoint proteins present on the surface of cells. Immune checkpoint inhibitors are emerging as a front-line treatment for several types of cancer.
Enadenotucirev is an investigational oncolytic virus that is in clinical trials for various cancers.
Akseli Hemminki July 27, 1973 (Helsinki) is a Finnish specialist in Oncology and Radiotherapy, Professor of Oncology and founder of two biotechnology companies.
Checkpoint inhibitor therapy is a form of cancer immunotherapy. The therapy targets immune checkpoints, key regulators of the immune system that when stimulated can dampen the immune response to an immunologic stimulus. Some cancers can protect themselves from attack by stimulating immune checkpoint targets. Checkpoint therapy can block inhibitory checkpoints, restoring immune system function. The first anti-cancer drug targeting an immune checkpoint was ipilimumab, a CTLA4 blocker approved in the United States in 2011.
SillaJen, Inc. is a South Korea-based biotechnology company, with offices in Busan, Yangsan and Seoul, South Korea, and San Francisco, California.
Passive antibody therapy, also called serum therapy, is a subtype of passive immunotherapy that administers antibodies to target and kill pathogens or cancer cells. It is designed to draw support from foreign antibodies that are donated from a person, extracted from animals, or made in the laboratory to elicit an immune response instead of relying on the innate immune system to fight disease. It has a long history from the 18th century for treating infectious diseases and is now a common cancer treatment. The mechanism of actions include: antagonistic and agonistic reaction, complement-dependent cytotoxicity (CDC), and antibody-dependent cellular cytotoxicity (ADCC).
Oligoclonal antibodies are an emerging immunological treatment relying on the combinatory use of several monoclonal antibodies (mAb) in one single drug. The composition can be made of mAb targeting different epitopes of a same protein (homo-combination) or mAb targeting different proteins (hetero-combination). It mimicks the natural polyclonal humoral immunological response to get better efficiency of the treatment. This strategy is most efficient in infections and in cancer treatment as it allow to overcome acquired resistance by pathogens and the plasticity of cancers.



