
A neurotransmitter is a signaling molecule secreted by a neuron to affect another cell across a synapse. The cell receiving the signal, or target cell, may be another neuron, but could also be a gland or muscle cell.

L-Tyrosine or tyrosine or 4-hydroxyphenylalanine is one of the 20 standard amino acids that are used by cells to synthesize proteins. It is a conditionally essential amino acid with a polar side group. The word "tyrosine" is from the Greek tyrós, meaning cheese, as it was first discovered in 1846 by German chemist Justus von Liebig in the protein casein from cheese. It is called tyrosyl when referred to as a functional group or side chain. While tyrosine is generally classified as a hydrophobic amino acid, it is more hydrophilic than phenylalanine. It is encoded by the codons UAC and UAU in messenger RNA.

Tryptophan (symbol Trp or W) is an α-amino acid that is used in the biosynthesis of proteins. Tryptophan contains an α-amino group, an α-carboxylic acid group, and a side chain indole, making it a polar molecule with a non-polar aromatic beta carbon substituent. Tryptophan is also a precursor to the neurotransmitter serotonin, the hormone melatonin, and vitamin B3 (niacin). It is encoded by the codon UGG.

Tryptamine is an indolamine metabolite of the essential amino acid tryptophan. The chemical structure is defined by an indole—a fused benzene and pyrrole ring, and a 2-aminoethyl group at the second carbon. The structure of tryptamine is a shared feature of certain aminergic neuromodulators including melatonin, serotonin, bufotenin and psychedelic derivatives such as dimethyltryptamine (DMT), psilocybin, psilocin and others.
A biogenic amine is a biogenic substance with one or more amine groups. They are basic nitrogenous compounds formed mainly by decarboxylation of amino acids or by amination and transamination of aldehydes and ketones. Biogenic amines are organic bases with low molecular weight and are synthesized by microbial, vegetable and animal metabolisms. In food and beverages they are formed by the enzymes of raw material or are generated by microbial decarboxylation of amino acids.

Aromatic L-amino acid decarboxylase, also known as DOPA decarboxylase (DDC), tryptophan decarboxylase, and 5-hydroxytryptophan decarboxylase, is a lyase enzyme, located in region 7p12.2-p12.1.
Shikimic acid, more commonly known as its anionic form shikimate, is a cyclohexene, a cyclitol and a cyclohexanecarboxylic acid. It is an important biochemical metabolite in plants and microorganisms. Its name comes from the Japanese flower shikimi, from which it was first isolated in 1885 by Johan Fredrik Eykman. The elucidation of its structure was made nearly 50 years later.
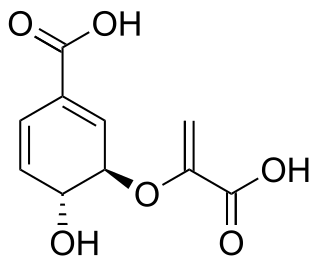
Chorismic acid, more commonly known as its anionic form chorismate, is an important biochemical intermediate in plants and microorganisms. It is a precursor for:

Indole alkaloids are a class of alkaloids containing a structural moiety of indole; many indole alkaloids also include isoprene groups and are thus called terpene indole or secologanin tryptamine alkaloids. Containing more than 4100 known different compounds, it is one of the largest classes of alkaloids. Many of them possess significant physiological activity and some of them are used in medicine. The amino acid tryptophan is the biochemical precursor of indole alkaloids.

Tryptophan hydroxylase (TPH) is an enzyme (EC 1.14.16.4) involved in the synthesis of the monoamine neurotransmitter serotonin. Tyrosine hydroxylase, phenylalanine hydroxylase, and tryptophan hydroxylase together constitute the family of biopterin-dependent aromatic amino acid hydroxylases. TPH catalyzes the following chemical reaction

Aralkylamine N-acetyltransferase (AANAT), also known as arylalkylamine N-acetyltransferase or serotonin N-acetyltransferase (SNAT), is an enzyme that is involved in the day/night rhythmic production of melatonin, by modification of serotonin. It is in humans encoded by the ~2.5 kb AANAT gene containing four exons, located on chromosome 17q25. The gene is translated into a 23 kDa large enzyme. It is well conserved through evolution and the human form of the protein is 80 percent identical to sheep and rat AANAT. It is an acetyl-CoA-dependent enzyme of the GCN5-related family of N-acetyltransferases (GNATs). It may contribute to multifactorial genetic diseases such as altered behavior in sleep/wake cycle and research is on-going with the aim of developing drugs that regulate AANAT function.

An aromatic amino acid is an amino acid that includes an aromatic ring.

Camptothecin (CPT) is a topoisomerase inhibitor. It was discovered in 1966 by M. E. Wall and M. C. Wani in systematic screening of natural products for anticancer drugs. It was isolated from the bark and stem of Camptotheca acuminata, a tree native to China used in traditional Chinese medicine. It has been used clinically in China for the treatment of gastrointestinal tumors. CPT showed anticancer activity in preliminary clinical trials, especially against breast, ovarian, colon, lung, and stomach cancers. However, it has low solubility and adverse effects have been reported when used therapeutically, so synthetic and medicinal chemists have developed numerous syntheses of camptothecin and various derivatives to increase the benefits of the chemical, with good results. Four CPT analogues have been approved and are used in cancer chemotherapy today: topotecan, irinotecan, belotecan, and trastuzumab deruxtecan. Camptothecin has also been found in other plants including Chonemorpha fragrans.
In enzymology, an indole 2,3-dioxygenase (EC 1.13.11.17) is an enzyme that catalyzes the chemical reaction

The enzyme anthranilate synthase catalyzes the chemical reaction

Tryptophan hydroxylase 1 (TPH1) is an isoenzyme of tryptophan hydroxylase which in humans is encoded by the TPH1 gene.

Hypertryptophanemia is a rare autosomal recessive metabolic disorder that results in a massive buildup of the amino acid tryptophan in the blood, with associated symptoms and tryptophanuria.

Indole is an organic compound with the formula C6H4CCNH3. Indole is classified as an aromatic heterocycle. It has a bicyclic structure, consisting of a six-membered benzene ring fused to a five-membered pyrrole ring. Indoles are derivatives of indole where one or more of the hydrogen atoms have been replaced by substituent groups. Indoles are widely distributed in nature, most notably as amino acid tryptophan and neurotransmitter serotonin.

L-Tryptophan decarboxylase is an enzyme distinguished by the substrate L-tryptophan.

A neurotransmitter prodrug, or neurotransmitter precursor, is a drug that acts as a prodrug of a neurotransmitter. A variety of neurotransmitter prodrugs have been developed and used in medicine. They can be useful when the neurotransmitter itself is not suitable for use as a pharmaceutical drug owing to unfavorable pharmacokinetic or physicochemical properties, for instance high susceptibility to metabolism, short elimination half-life, or lack of blood–brain barrier permeability. Besides their use in medicine, neurotransmitter prodrugs have also been used as recreational drugs in some cases.


















