Related Research Articles

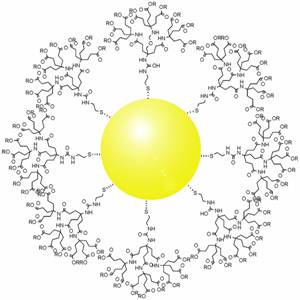
Dendrimers are highly ordered, branched polymeric molecules. Synonymous terms for dendrimer include arborols and cascade molecules. Typically, dendrimers are symmetric about the core, and often adopt a spherical three-dimensional morphology. The word dendron is also encountered frequently. A dendron usually contains a single chemically addressable group called the focal point or core. The difference between dendrons and dendrimers is illustrated in the top figure, but the terms are typically encountered interchangeably.
Sarepta Therapeutics, Inc. is a medical research and drug development company with corporate offices and research facilities in Cambridge, Massachusetts, United States. Incorporated in 1980 as AntiVirals, shortly before going public the company changed its name from AntiVirals to AVI BioPharma soon with stock symbol AVII and in July 2012 changed name from AVI BioPharma to Sarepta Therapeutics and SRPT respectively. As of the end of 2019, the company has two approved drugs.
The enhanced permeability and retention (EPR) effect is a controversial concept by which molecules of certain sizes tend to accumulate in tumor tissue much more than they do in normal tissues. The general explanation that is given for this phenomenon is that, in order for tumor cells to grow quickly, they must stimulate the production of blood vessels. VEGF and other growth factors are involved in cancer angiogenesis. Tumor cell aggregates as small as 150–200 μm, start to become dependent on blood supply carried out by neovasculature for their nutritional and oxygen supply. These newly formed tumor vessels are usually abnormal in form and architecture. They are poorly aligned defective endothelial cells with wide fenestrations, lacking a smooth muscle layer, or innervation with a wider lumen, and impaired functional receptors for angiotensin II. Furthermore, tumor tissues usually lack effective lymphatic drainage. All of these factors lead to abnormal molecular and fluid transport dynamics, especially for macromolecular drugs. This phenomenon is referred to as the "enhanced permeability and retention (EPR) effect" of macromolecules and lipids in solid tumors. The EPR effect is further enhanced by many pathophysiological factors involved in enhancement of the extravasation of macromolecules in solid tumor tissues. For instance, bradykinin, nitric oxide / peroxynitrite, prostaglandins, vascular permeability factor, tumor necrosis factor and others. One factor that leads to the increased retention is the lack of lymphatics around the tumor region which would filter out such particles under normal conditions.
CRLX101 is a novel approach to cancer chemotherapy that is under investigation in human trials. It is an example of a nanomedicine.

Paclitaxel trevatide is an experimental chemotherapy drug that is under development by Angiochem Inc, a Canadian biotech company. Phase II clinical trials have completed for several indications, and the company is preparing for phase III trials.

Solid lipid nanoparticles, or lipid nanoparticles (LNPs), are nanoparticles composed of lipids. They are a novel pharmaceutical drug delivery system, and a novel pharmaceutical formulation. LNPs as a drug delivery vehicle were first approved in 2018 for the siRNA drug Onpattro. LNPs became more widely known in late 2020, as some COVID-19 vaccines that use RNA vaccine technology coat the fragile mRNA strands with PEGylated lipid nanoparticles as their delivery vehicle.

Plus Therapeutics, Inc. is a clinical-stage pharmaceutical company developing innovative, targeted radiotherapeutics for adults and children with rare and difficult-to-treat cancers. The company is headquartered in Austin, Texas, United States.
Glembatumumab vedotin is an antibody-drug conjugate (ADC) that targets cancer cells expressing transmembrane glycoprotein NMB (GPNMB).

Jindřich Henry Kopeček was born in Strakonice, Czech Republic, as the son of Jan and Herta Zita (Krombholz) Kopeček. He is distinguished professor of pharmaceutical chemistry and distinguished professor of biomedical engineering at the University of Utah in Salt Lake City, Utah. Kopeček is also an honorary professor at Sichuan University in Chengdu, China. His research focuses on biorecognition of macromolecules, bioconjugate chemistry, drug delivery systems, self-assembled biomaterials, and drug-free macromolecular therapeutics.

N-(2-Hydroxypropyl)methacrylamide or HPMA is the monomer used to make the polymer poly(N- methacrylamide).
AbbVie is an American publicly traded biopharmaceutical company founded in 2013. It originated as a spin-off of Abbott Laboratories.
Polymer-drug conjugates are nano-medicine products under development for cancer diagnosis and treatment. There are more than 10 anticancer conjugates in clinical development. Polymer-drug conjugates are drug molecules held in polymer molecules, which act as the delivery system for the drug. Polymer drugs have passed multidrug resistance (MDR) testing and hence may become a viable treatment for endocrine-related cancers. A cocktail of pendant drugs could be delivered by water-soluble polymer platforms. The physical and chemical properties of the polymers used in polymer-drug conjugates are specially synthesized to flow through the kidneys and liver without being filtered out, allowing the drugs to be used more effectively. Traditional polymers used in polymer-drug conjugates can be degraded through enzymatic activity and acidity. Polymers are now being synthesized to be sensitive to specific enzymes that are apparent in diseased tissue. The drugs remain attached to the polymer and are not activated until the enzymes associated with the diseased tissue are present. This process significantly minimizes damage to healthy tissue.
Mark E. Davis is the Warren and Katherine Schlinger Professor of Chemical Engineering at the California Institute of Technology. He is a member of the City of Hope National Medical Center. He earned his B.S., M.S., and Ph.D. in Chemical Engineering all from the University of Kentucky. His lab focuses on the synthesis of materials for catalysis and biocompatible materials for drug delivery.
Nektar Therapeutics (Nektar) is an American biopharmaceutical company. The company was founded in 1990 and is based in San Francisco, California. The company develops new drug candidates by applying its proprietary PEGylation and advanced polymer conjugate technologies to modify chemical structure of substances. It is a technology supplier to a number of pharmaceutical companies including Affymax, Amgen, Merck, Pfizer and UCB Pharma, etc. The company developed the world's first inhalable non-injectable insulin, Exubera, which was awarded as the bronze award by Wall Street Journal for its technological breakthrough.

Ram I. Mahato is a professor and chairman of the Department of Pharmaceutical Sciences, University of Nebraska Medical Center, Omaha, United States. He was Professor of Pharmaceutical Sciences and Biomedical Engineering at the University of Tennessee Health Science Center, Memphis. He was Research Assistant Professor at the University of Utah, Senior Scientist at GeneMedicine Inc and a postdoctoral fellow at the University of Southern California in Los Angeles. Washington University in St. Louis, and Kyoto University, Kyoto, Japan. He received a PhD in drug delivery from the University of Strathclyde, UK and BS from China Pharmaceutical University, Nanjing. He is a CRS Fellow, AAPS Fellow, Permanent Member of BTSS/NIH Study section (2009–2013), and ASGCT Scientific Advisor.
Gold nanoparticles in chemotherapy and radiotherapy is the use of colloidal gold in therapeutic treatments, often for cancer or arthritis. Gold nanoparticle technology shows promise in the advancement of cancer treatments. Some of the properties that gold nanoparticles possess, such as small size, non-toxicity and non-immunogenicity make these molecules useful candidates for targeted drug delivery systems. With tumor-targeting delivery vectors becoming smaller, the ability to by-pass the natural barriers and obstacles of the body becomes more probable. To increase specificity and likelihood of drug delivery, tumor specific ligands may be grafted onto the particles along with the chemotherapeutic drug molecules, to allow these molecules to circulate throughout the tumor without being redistributed into the body.

Optimer ligands are short synthetic oligonucleotide molecules composed of DNA or RNA that bind to a specific target molecule. They are engineered to bind their target molecules with affinity typically in the low nanomolar range. Optimers can be used as antibody mimetics in a range of applications, and have been optimized to increase their stability, reduce their molecular weight, and offer increased scalability and consistency in manufacture compared to standard aptamer molecules.
Nanoparticle drug delivery systems are engineered technologies that use nanoparticles for the targeted delivery and controlled release of therapeutic agents. The modern form of a drug delivery system should minimize side-effects and reduce both dosage and dosage frequency. Recently, nanoparticles have aroused attention due to their potential application for effective drug delivery.
Hamid Ghandehari is an Iranian-American drug delivery research scientist, and a professor in the Departments of Pharmaceutics and Pharmaceutical Chemistry and Biomedical Engineering at the University of Utah. His research is focused in recombinant polymers for drug and gene delivery, nanotoxicology of dendritic and inorganic constructs, water-soluble polymers for targeted delivery and poly(amidoamine) dendrimers for oral delivery.
Jianjun Cheng is a Chinese material scientist.
References
- ↑ "Insert Therapeutics, Inc". ctventures.com. Retrieved 1 February 2018.
- 1 2 3 IT-101 trial