In particle physics, an elementary particle or fundamental particle is a subatomic particle that is not composed of other particles. Particles currently thought to be elementary include electrons, the fundamental fermions, as well as the fundamental bosons, which generally are force particles that mediate interactions among fermions. A particle containing two or more elementary particles is a composite particle.

The neutron is a subatomic particle, symbol
n
or
n0
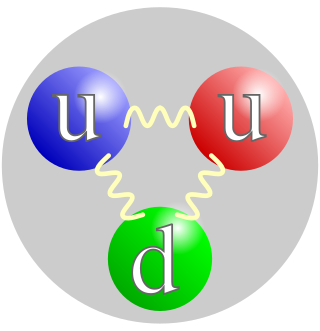
, which has a neutral charge, and a mass slightly greater than that of a proton. Protons and neutrons constitute the nuclei of atoms. Since protons and neutrons behave similarly within the nucleus, and each has a mass of approximately one atomic mass unit, they are both referred to as nucleons. Their properties and interactions are described by nuclear physics. Protons and neutrons are not elementary particles; each is composed of three quarks.

Nuclear physics is the field of physics that studies atomic nuclei and their constituents and interactions, in addition to the study of other forms of nuclear matter.

In physics and chemistry, a nucleon is either a proton or a neutron, considered in its role as a component of an atomic nucleus. The number of nucleons in a nucleus defines the atom's mass number.

Nuclear fission is a reaction in which the nucleus of an atom splits into two or more smaller nuclei. The fission process often produces gamma photons, and releases a very large amount of energy even by the energetic standards of radioactive decay.

Particle physics or high energy physics is the study of fundamental particles and forces that constitute matter and radiation. The fundamental particles in the universe are classified in the Standard Model as fermions and bosons. There are three generations of fermions, but ordinary matter is made only from the first fermion generation. The first generation consists of up and down quarks which form protons and neutrons, and electrons and electron neutrinos. The three fundamental interactions known to be mediated by bosons are electromagnetism, the weak interaction, and the strong interaction.
The strong interaction or strong force is a fundamental interaction that confines quarks into proton, neutron, and other hadron particles. The strong interaction also binds neutrons and protons to create atomic nuclei, where it is called the nuclear force.
In physical sciences, a subatomic particle is a particle that composes an atom. According to the Standard Model of particle physics, a subatomic particle can be either a composite particle, which is composed of other particles, or an elementary particle, which is not composed of other particles. Particle physics and nuclear physics study these particles and how they interact.

In nuclear physics and nuclear chemistry, a nuclear reaction is a process in which two nuclei, or a nucleus and an external subatomic particle, collide to produce one or more new nuclides. Thus, a nuclear reaction must cause a transformation of at least one nuclide to another. If a nucleus interacts with another nucleus or particle and they then separate without changing the nature of any nuclide, the process is simply referred to as a type of nuclear scattering, rather than a nuclear reaction.

The Joint Institute for Nuclear Research, in Dubna, Moscow Oblast, Russia, is an international research center for nuclear sciences, with 5500 staff members including 1200 researchers holding over 1000 Ph.Ds from eighteen countries. Most scientists, however, are eminent Russian scientists.

The nuclear force is a force that acts between the protons and neutrons of atoms. Neutrons and protons, both nucleons, are affected by the nuclear force almost identically. Since protons have charge +1 e, they experience an electric force that tends to push them apart, but at short range the attractive nuclear force is strong enough to overcome the electromagnetic force. The nuclear force binds nucleons into atomic nuclei.
Dmitri Dmitrievich Ivanenko was a Ukrainian theoretical physicist who made great contributions to the physical science of the twentieth century, especially to nuclear physics, field theory, and gravitation theory. He worked in the Poltava Gravimetric Observatory of the Institute of Geophysics of NAS of Ukraine, was the head of the Theoretical Department Ukrainian Physico-Technical Institute in Kharkiv, Head of the Department of Theoretical Physics of the Kharkiv Institute of Mechanical Engineering. Professor of University of Kharkiv, Professor of Moscow State University.
The A.I. Alikhanyan National Science Laboratory is a research institute located in Yerevan, Armenia. It was founded in 1943 as a branch of the Yerevan State University by brothers Abram Alikhanov and Artem Alikhanian. It was often referred to by the acronym YerPhI. In 2011 it was renamed to its current name A.I. Alikhanyan National Science Laboratory.

Understanding the structure of the atomic nucleus is one of the central challenges in nuclear physics.

Igal Talmi is an Israeli nuclear physicist.

In classical physics and general chemistry, matter is any substance that has mass and takes up space by having volume. All everyday objects that can be touched are ultimately composed of atoms, which are made up of interacting subatomic particles, and in everyday as well as scientific usage, "matter" generally includes atoms and anything made up of them, and any particles that act as if they have both rest mass and volume. However it does not include massless particles such as photons, or other energy phenomena or waves such as light or heat. Matter exists in various states. These include classical everyday phases such as solid, liquid, and gas – for example water exists as ice, liquid water, and gaseous steam – but other states are possible, including plasma, Bose–Einstein condensates, fermionic condensates, and quark–gluon plasma.

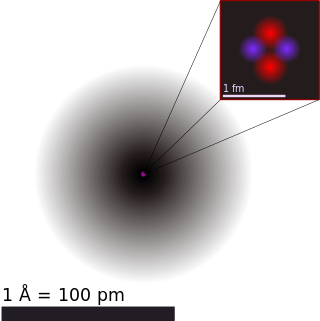
The atomic nucleus is the small, dense region consisting of protons and neutrons at the center of an atom, discovered in 1911 by Ernest Rutherford based on the 1909 Geiger–Marsden gold foil experiment. After the discovery of the neutron in 1932, models for a nucleus composed of protons and neutrons were quickly developed by Dmitri Ivanenko and Werner Heisenberg. An atom is composed of a positively charged nucleus, with a cloud of negatively charged electrons surrounding it, bound together by electrostatic force. Almost all of the mass of an atom is located in the nucleus, with a very small contribution from the electron cloud. Protons and neutrons are bound together to form a nucleus by the nuclear force.

The idea that matter consists of smaller particles and that there exists a limited number of sorts of primary, smallest particles in nature has existed in natural philosophy at least since the 6th century BC. Such ideas gained physical credibility beginning in the 19th century, but the concept of "elementary particle" underwent some changes in its meaning: notably, modern physics no longer deems elementary particles indestructible. Even elementary particles can decay or collide destructively; they can cease to exist and create (other) particles in result.
The discovery of the neutron and its properties was central to the extraordinary developments in atomic physics in the first half of the 20th century. Early in the century, Ernest Rutherford developed a crude model of the atom, based on the gold foil experiment of Hans Geiger and Ernest Marsden. In this model, atoms had their mass and positive electric charge concentrated in a very small nucleus. By 1920, isotopes of chemical elements had been discovered, the atomic masses had been determined to be (approximately) integer multiples of the mass of the hydrogen atom, and the atomic number had been identified as the charge on the nucleus. Throughout the 1920s, the nucleus was viewed as composed of combinations of protons and electrons, the two elementary particles known at the time, but that model presented several experimental and theoretical contradictions.