A coordination complex is a chemical compound consisting of a central atom or ion, which is usually metallic and is called the coordination centre, and a surrounding array of bound molecules or ions, that are in turn known as ligands or complexing agents. Many metal-containing compounds, especially those that include transition metals, are coordination complexes.

Inorganic chemistry deals with synthesis and behavior of inorganic and organometallic compounds. This field covers chemical compounds that are not carbon-based, which are the subjects of organic chemistry. The distinction between the two disciplines is far from absolute, as there is much overlap in the subdiscipline of organometallic chemistry. It has applications in every aspect of the chemical industry, including catalysis, materials science, pigments, surfactants, coatings, medications, fuels, and agriculture.
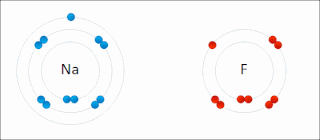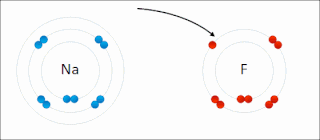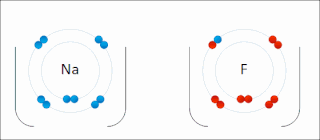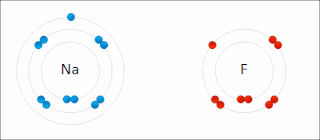
Ionic bonding is a type of chemical bonding that involves the electrostatic attraction between oppositely charged ions, or between two atoms with sharply different electronegativities, and is the primary interaction occurring in ionic compounds. It is one of the main types of bonding, along with covalent bonding and metallic bonding. Ions are atoms with an electrostatic charge. Atoms that gain electrons make negatively charged ions. Atoms that lose electrons make positively charged ions. This transfer of electrons is known as electrovalence in contrast to covalence. In the simplest case, the cation is a metal atom and the anion is a nonmetal atom, but these ions can be more complex, e.g. molecular ions like NH+
4 or SO2−
4. In simpler words, an ionic bond results from the transfer of electrons from a metal to a non-metal to obtain a full valence shell for both atoms.
The lanthanide or lanthanoid series of chemical elements comprises at least the 14 metallic chemical elements with atomic numbers 57–70, from lanthanum through ytterbium. In the periodic table, they fill the 4f orbitals. Lutetium is also sometimes considered a lanthanide, despite being a d-block element and a transition metal.
In chemistry, a transition metal is a chemical element in the d-block of the periodic table, though the elements of group 12 are sometimes excluded. The lanthanide and actinide elements are called inner transition metals and are sometimes considered to be transition metals as well.

In organic chemistry, an aldehyde is an organic compound containing a functional group with the structure R−CH=O. The functional group itself can be referred to as an aldehyde but can also be classified as a formyl group. Aldehydes are a common motif in many chemicals important in technology and biology.
In chemistry, the oxidation state, or oxidation number, is the hypothetical charge of an atom if all of its bonds to other atoms were fully ionic. It describes the degree of oxidation of an atom in a chemical compound. Conceptually, the oxidation state may be positive, negative or zero. Beside nearly-pure ionic bonding, many covalent bonds exhibit a strong ionicity, making oxidation state a useful predictor of charge.

Redox is a type of chemical reaction in which the oxidation states of the reactants change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a decrease in the oxidation state. The oxidation and reduction processes occur simultaneously in the chemical reaction.
In chemistry, π backbonding is a π-bonding interaction between a filled (or half filled) orbital of a transition metal atom and a vacant orbital on an adjacent ion or molecule. In this type of interaction, electrons from the metal are used to bond to the ligand, which dissipates excess negative charge and stabilizes the metal. It is common in transition metals with low oxidation states that have ligands such as carbon monoxide, olefins, or phosphines. The ligands involved in π backbonding can be broken into three groups: carbonyls and nitrogen analogs, alkenes and alkynes, and phosphines. Compounds where π backbonding is prominent include Ni(CO)4, Zeise's salt, and molybdenum and iron dinitrogen complexes.
In chemistry, the valence or valency of an atom is a measure of its combining capacity with other atoms when it forms chemical compounds or molecules. Valence is generally understood to be the number of chemical bonds that each atom of a given chemical element typically forms. Double bonds are considered to be two bonds, triple bonds to be three, quadruple bonds to be four, quintuple bonds to be five and sextuple bonds to be six. In most compounds, the valence of hydrogen is 1, of oxygen is 2, of nitrogen is 3, and of carbon is 4. Valence is not to be confused with the related concepts of the coordination number, the oxidation state, or the number of valence electrons for a given atom.
The nitrosonium ion is NO+, in which the nitrogen atom is bonded to an oxygen atom with a bond order of 3, and the overall diatomic species bears a positive charge. It can be viewed as nitric oxide with one electron removed. This ion is usually obtained as the following salts: NOClO4, NOSO4H (nitrosylsulfuric acid, more descriptively written ONSO3OH) and NOBF4. The ClO−4 and BF−4 salts are slightly soluble in acetonitrile CH3CN. NOBF4 can be purified by sublimation at 200–250 °C and 0.01 mmHg (1.3 Pa).
Inner sphere electron transfer or bonded electron transfer is a redox chemical reaction that proceeds via a covalent linkage—a strong electronic interaction—between the oxidant and the reductant reactants. In inner sphere electron transfer, a ligand bridges the two metal redox centers during the electron transfer event. Inner sphere reactions are inhibited by large ligands, which prevent the formation of the crucial bridged intermediate. Thus, inner sphere ET is rare in biological systems, where redox sites are often shielded by bulky proteins. Inner sphere ET is usually used to describe reactions involving transition metal complexes and most of this article is written from this perspective. However, redox centers can consist of organic groups rather than metal centers.
Outer sphere refers to an electron transfer (ET) event that occurs between chemical species that remain separate and intact before, during, and after the ET event. In contrast, for inner sphere electron transfer the participating redox sites undergoing ET become connected by a chemical bridge. Because the ET in outer sphere electron transfer occurs between two non-connected species, the electron is forced to move through space from one redox center to the other.

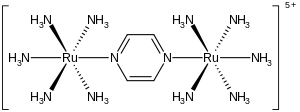
The Creutz–Taube ion is the metal complex with the formula {[Ru(NH3)5]2(C4H4N2)}5+. This cationic species has been heavily studied in an effort to understand the intimate details of inner sphere electron transfer, that is, how electrons move from one metal complex to another. The ion is named after Carol Creutz, who first prepared the complex, and her thesis advisor Henry Taube, who received a Nobel Prize in Chemistry for this and related discoveries on electron transfer.
In X-ray absorption spectroscopy, the K-edge is a sudden increase in x-ray absorption occurring when the energy of the X-rays is just above the binding energy of the innermost electron shell of the atoms interacting with the photons. The term is based on X-ray notation, where the innermost electron shell is known as the K-shell. Physically, this sudden increase in attenuation is caused by the photoelectric absorption of the photons. For this interaction to occur, the photons must have more energy than the binding energy of the K-shell electrons (K-edge). A photon having an energy just above the binding energy of the electron is therefore more likely to be absorbed than a photon having an energy just below this binding energy or significantly above it.
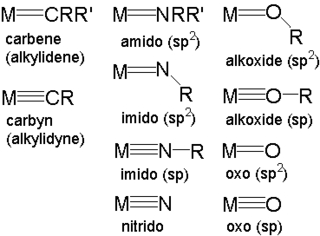
In organometallic chemistry, a metal–ligand multiple bond describes the interaction of certain ligands with a metal with a bond order greater than one. Coordination complexes featuring multiply bonded ligands are of both scholarly and practical interest. transition metal carbene complexes catalyze the olefin metathesis reaction. Metal oxo intermediates are pervasive in oxidation catalysis.
Equilibrium chemistry is concerned with systems in chemical equilibrium. The unifying principle is that the free energy of a system at equilibrium is the minimum possible, so that the slope of the free energy with respect to the reaction coordinate is zero. This principle, applied to mixtures at equilibrium provides a definition of an equilibrium constant. Applications include acid–base, host–guest, metal–complex, solubility, partition, chromatography and redox equilibria.

Rhodocene is a chemical compound with the formula [Rh(C5H5)2]. Each molecule contains an atom of rhodium bound between two planar aromatic systems of five carbon atoms known as cyclopentadienyl rings in a sandwich arrangement. It is an organometallic compound as it has (haptic) covalent rhodium–carbon bonds. The [Rh(C5H5)2] radical is found above 150 °C (302 °F) or when trapped by cooling to liquid nitrogen temperatures (−196 °C [−321 °F]). At room temperature, pairs of these radicals join via their cyclopentadienyl rings to form a dimer, a yellow solid.
Transition metal oxides are compounds composed of oxygen atoms bound to transition metals. They are commonly utilized for their catalytic activity and semiconducting properties. Transition metal oxides are also frequently used as pigments in paints and plastics, most notably titanium dioxide. Transition metal oxides have a wide variety of surface structures which affect the surface energy of these compounds and influence their chemical properties. The relative acidity and basicity of the atoms present on the surface of metal oxides are also affected by the coordination of the metal cation and oxygen anion, which alter the catalytic properties of these compounds. For this reason, structural defects in transition metal oxides greatly influence their catalytic properties. The acidic and basic sites on the surface of metal oxides are commonly characterized via infrared spectroscopy, calorimetry among other techniques. Transition metal oxides can also undergo photo-assisted adsorption and desorption that alter their electrical conductivity. One of the more researched properties of these compounds is their response to electromagnetic radiation, which makes them useful catalysts for redox reactions, isotope exchange and specialized surfaces.

Mixed valence complexes contain an element which is present in more than one oxidation state. Well-known mixed valence compounds include the Creutz–Taube complex, Prussian blue, and molybdenum blue. Many solids are mixed-valency including indium chalcogenides.