Related Research Articles

Combustion, or burning, is a high-temperature exothermic redox chemical reaction between a fuel and an oxidant, usually atmospheric oxygen, that produces oxidized, often gaseous products, in a mixture termed as smoke. Combustion does not always result in fire, because a flame is only visible when substances undergoing combustion vaporize, but when it does, a flame is a characteristic indicator of the reaction. While activation energy must be supplied to initiate combustion, the heat from a flame may provide enough energy to make the reaction self-sustaining. The study of combustion is known as combustion science.

In physics, physical chemistry and engineering, fluid dynamics is a subdiscipline of fluid mechanics that describes the flow of fluids — liquids and gases. It has several subdisciplines, including aerodynamics and hydrodynamics. Fluid dynamics has a wide range of applications, including calculating forces and moments on aircraft, determining the mass flow rate of petroleum through pipelines, predicting weather patterns, understanding nebulae in interstellar space and modelling fission weapon detonation.

Stoichiometry is the relationship between the weights of reactants and products before, during, and following chemical reactions.

Computational fluid dynamics (CFD) is a branch of fluid mechanics that uses numerical analysis and data structures to analyze and solve problems that involve fluid flows. Computers are used to perform the calculations required to simulate the free-stream flow of the fluid, and the interaction of the fluid with surfaces defined by boundary conditions. With high-speed supercomputers, better solutions can be achieved, and are often required to solve the largest and most complex problems. Ongoing research yields software that improves the accuracy and speed of complex simulation scenarios such as transonic or turbulent flows. Initial validation of such software is typically performed using experimental apparatus such as wind tunnels. In addition, previously performed analytical or empirical analysis of a particular problem can be used for comparison. A final validation is often performed using full-scale testing, such as flight tests.

Nonlinear dimensionality reduction, also known as manifold learning, is any of various related techniques that aim to project high-dimensional data onto lower-dimensional latent manifolds, with the goal of either visualizing the data in the low-dimensional space, or learning the mapping itself. The techniques described below can be understood as generalizations of linear decomposition methods used for dimensionality reduction, such as singular value decomposition and principal component analysis.
Chemical kinetics, also known as reaction kinetics, is the branch of physical chemistry that is concerned with understanding the rates of chemical reactions. It is different from chemical thermodynamics, which deals with the direction in which a reaction occurs but in itself tells nothing about its rate. Chemical kinetics includes investigations of how experimental conditions influence the speed of a chemical reaction and yield information about the reaction's mechanism and transition states, as well as the construction of mathematical models that also can describe the characteristics of a chemical reaction.
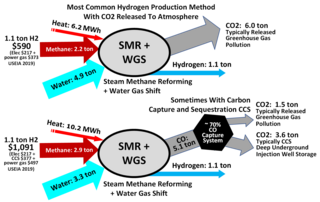
Steam reforming or steam methane reforming (SMR) is a method for producing syngas (hydrogen and carbon monoxide) by reaction of hydrocarbons with water. Commonly natural gas is the feedstock. The main purpose of this technology is hydrogen production. The reaction is represented by this equilibrium:

A potential energy surface (PES) or energy landscape describes the energy of a system, especially a collection of atoms, in terms of certain parameters, normally the positions of the atoms. The surface might define the energy as a function of one or more coordinates; if there is only one coordinate, the surface is called a potential energy curve or energy profile. An example is the Morse/Long-range potential.
In quantum chemistry, a conical intersection of two or more potential energy surfaces is the set of molecular geometry points where the potential energy surfaces are degenerate (intersect) and the non-adiabatic couplings between these states are non-vanishing. In the vicinity of conical intersections, the Born–Oppenheimer approximation breaks down and the coupling between electronic and nuclear motion becomes important, allowing non-adiabatic processes to take place. The location and characterization of conical intersections are therefore essential to the understanding of a wide range of important phenomena governed by non-adiabatic events, such as photoisomerization, photosynthesis, vision and the photostability of DNA. The conical intersection involving the ground electronic state potential energy surface of the C6H3F3+ molecular ion is discussed in connection with the Jahn–Teller effect in Section 13.4.2 on pages 380-388 of the textbook by Bunker and Jensen.

Enzyme kinetics is the study of the rates of enzyme-catalysed chemical reactions. In enzyme kinetics, the reaction rate is measured and the effects of varying the conditions of the reaction are investigated. Studying an enzyme's kinetics in this way can reveal the catalytic mechanism of this enzyme, its role in metabolism, how its activity is controlled, and how a drug or a modifier might affect the rate.

Reaction–diffusion systems are mathematical models that correspond to several physical phenomena. The most common is the change in space and time of the concentration of one or more chemical substances: local chemical reactions in which the substances are transformed into each other, and diffusion which causes the substances to spread out over a surface in space.
Dissipative solitons (DSs) are stable solitary localized structures that arise in nonlinear spatially extended dissipative systems due to mechanisms of self-organization. They can be considered as an extension of the classical soliton concept in conservative systems. An alternative terminology includes autosolitons, spots and pulses.
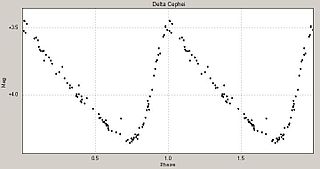
Stellar pulsations are caused by expansions and contractions in the outer layers as a star seeks to maintain equilibrium. These fluctuations in stellar radius cause corresponding changes in the luminosity of the star. Astronomers are able to deduce this mechanism by measuring the spectrum and observing the Doppler effect. Many intrinsic variable stars that pulsate with large amplitudes, such as the classical Cepheids, RR Lyrae stars and large-amplitude Delta Scuti stars show regular light curves.
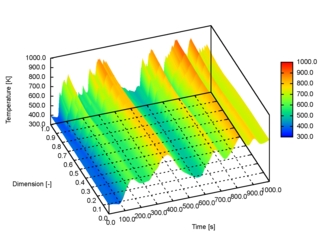
The extended discrete element method (XDEM) is a numerical technique that extends the dynamics of granular material or particles as described through the classical discrete element method (DEM) by additional properties such as the thermodynamic state, stress/strain or electro-magnetic field for each particle. Contrary to a continuum mechanics concept, the XDEM aims at resolving the particulate phase with its various processes attached to the particles. While the discrete element method predicts position and orientation in space and time for each particle, the extended discrete element method additionally estimates properties such as internal temperature and/or species distribution or mechanical impact with structures.
In mathematics, inertial manifolds are concerned with the long term behavior of the solutions of dissipative dynamical systems. Inertial manifolds are finite-dimensional, smooth, invariant manifolds that contain the global attractor and attract all solutions exponentially quickly. Since an inertial manifold is finite-dimensional even if the original system is infinite-dimensional, and because most of the dynamics for the system takes place on the inertial manifold, studying the dynamics on an inertial manifold produces a considerable simplification in the study of the dynamics of the original system.
Combustion models for CFD refers to combustion models for computational fluid dynamics. Combustion is defined as a chemical reaction in which a hydrocarbon fuel reacts with an oxidant to form products, accompanied with the release of energy in the form of heat. Being the integral part of various engineering applications like: internal combustion engines, aircraft engines, rocket engines, furnaces, and power station combustors, combustion manifests itself as a wide domain during the design, analysis and performance characteristics stages of the above-mentioned applications. With the added complexity of chemical kinetics and achieving reacting flow mixture environment, proper modeling physics has to be incorporated during computational fluid dynamic (CFD) simulations of combustion. Hence the following discussion presents a general outline of the various adequate models incorporated with the Computational fluid dynamic code to model the process of combustion.
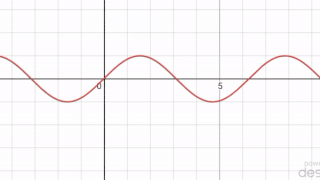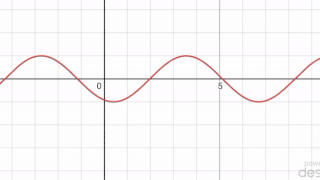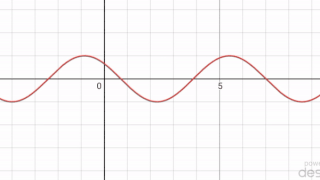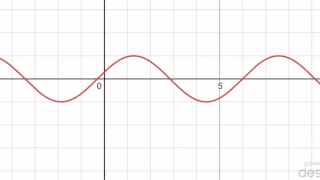
In mathematics, a periodic travelling wave is a periodic function of one-dimensional space that moves with constant speed. Consequently, it is a special type of spatiotemporal oscillation that is a periodic function of both space and time.
Autowaves are self-supporting non-linear waves in active media. The term is generally used in processes where the waves carry relatively low energy, which is necessary for synchronization or switching the active medium.

James Bernhard Anderson was an American chemist and physicist. From 1995 to 2014 he was Evan Pugh Professor of Chemistry and Physics at the Pennsylvania State University. He specialized in Quantum Chemistry by Monte Carlo methods, molecular dynamics of reactive collisions, kinetics and mechanisms of gas phase reactions, and rare-event theory.
Supersymmetric theory of stochastic dynamics or stochastics (STS) is an exact theory of stochastic (partial) differential equations (SDEs), the class of mathematical models with the widest applicability covering, in particular, all continuous time dynamical systems, with and without noise. The main utility of the theory from the physical point of view is a rigorous theoretical explanation of the ubiquitous spontaneous long-range dynamical behavior that manifests itself across disciplines via such phenomena as 1/f, flicker, and crackling noises and the power-law statistics, or Zipf's law, of instantonic processes like earthquakes and neuroavalanches. From the mathematical point of view, STS is interesting because it bridges the two major parts of mathematical physics – the dynamical systems theory and topological field theories. Besides these and related disciplines such as algebraic topology and supersymmetric field theories, STS is also connected with the traditional theory of stochastic differential equations and the theory of pseudo-Hermitian operators.
References
- ↑ Maas, U.; Pope, S. B. (1992). "Implementation of simplified chemical kinetics based on intrinsic low-dimensional manifolds" (PDF). Symposium (International) on Combustion. 24: 103–112. doi:10.1016/S0082-0784(06)80017-2.
- ↑ Maas, U.; Pope, S. B. (1992). "Simplifying chemical kinetics: Intrinsic low-dimensional manifolds in composition space" (PDF). Combustion and Flame. 88 (3–4): 239. doi:10.1016/0010-2180(92)90034-M.
- ↑ Bongers, H.; Van Oijen, J. A.; De Goey, L. P. H. (2002). "Intrinsic low-dimensional manifold method extended with diffusion". Proceedings of the Combustion Institute. 29: 1371–1378. doi:10.1016/S1540-7489(02)80168-7.
- ↑ Tomlin, A. S.; Whitehouse, L.; Lowe, R. (2002). "The Estimation of Intrinsic Low Dimensional Manifold Dimension in Atmospheric Chemical Reaction Systems". Air Pollution Modelling and Simulation. p. 245. doi:10.1007/978-3-662-04956-3_25. ISBN 978-3-642-07637-4.