
The photoelectric effect is the emission of electrons when electromagnetic radiation, such as light, hits a material. Electrons emitted in this manner are called photoelectrons. The phenomenon is studied in condensed matter physics, and solid state and quantum chemistry to draw inferences about the properties of atoms, molecules and solids. The effect has found use in electronic devices specialized for light detection and precisely timed electron emission.

Spectroscopy is the study of the interaction between matter and electromagnetic radiation as a function of the wavelength or frequency of the radiation. In simpler terms, spectroscopy is the precise study of color as generalized from visible light to all bands of the electromagnetic spectrum; indeed, historically, spectroscopy originated as the study of the wavelength dependence of the absorption by gas phase matter of visible light dispersed by a prism. Matter waves and acoustic waves can also be considered forms of radiative energy, and recently gravitational waves have been associated with a spectral signature in the context of the Laser Interferometer Gravitational-Wave Observatory (LIGO).

Auger electron spectroscopy is a common analytical technique used specifically in the study of surfaces and, more generally, in the area of materials science. Underlying the spectroscopic technique is the Auger effect, as it has come to be called, which is based on the analysis of energetic electrons emitted from an excited atom after a series of internal relaxation events. The Auger effect was discovered independently by both Lise Meitner and Pierre Auger in the 1920s. Though the discovery was made by Meitner and initially reported in the journal Zeitschrift für Physik in 1922, Auger is credited with the discovery in most of the scientific community. Until the early 1950s Auger transitions were considered nuisance effects by spectroscopists, not containing much relevant material information, but studied so as to explain anomalies in X-ray spectroscopy data. Since 1953 however, AES has become a practical and straightforward characterization technique for probing chemical and compositional surface environments and has found applications in metallurgy, gas-phase chemistry, and throughout the microelectronics industry.

X-ray photoelectron spectroscopy (XPS) is a surface-sensitive quantitative spectroscopic technique based on the photoelectric effect that can identify the elements that exist within a material or are covering its surface, as well as their chemical state, and the overall electronic structure and density of the electronic states in the material. XPS is a powerful measurement technique because it not only shows what elements are present, but also what other elements they are bonded to. The technique can be used in line profiling of the elemental composition across the surface, or in depth profiling when paired with ion-beam etching. It is often applied to study chemical processes in the materials in their as-received state or after cleavage, scraping, exposure to heat, reactive gasses or solutions, ultraviolet light, or during ion implantation.
Raman scattering or the Raman effect is the inelastic scattering of photons by matter, meaning that there is both an exchange of energy and a change in the light's direction. Typically this effect involves vibrational energy being gained by a molecule as incident photons from a visible laser are shifted to lower energy. This is called normal Stokes Raman scattering. The effect is exploited by chemists and physicists to gain information about materials for a variety of purposes by performing various forms of Raman spectroscopy. Many other variants of Raman spectroscopy allow rotational energy to be examined and electronic energy levels may be examined if an X-ray source is used in addition to other possibilities. More complex techniques involving pulsed lasers, multiple laser beams and so on are known.
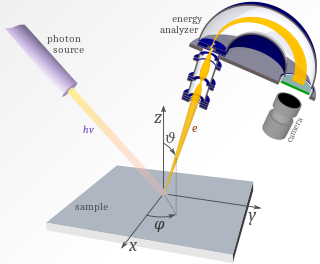
Photoemission spectroscopy (PES), also known as photoelectron spectroscopy, refers to energy measurement of electrons emitted from solids, gases or liquids by the photoelectric effect, in order to determine the binding energies of electrons in the substance. The term refers to various techniques, depending on whether the ionization energy is provided by X-ray, XUV or UV photons. Regardless of the incident photon beam, however, all photoelectron spectroscopy revolves around the general theme of surface analysis by measuring the ejected electrons.
Photoemission electron microscopy is a type of electron microscopy that utilizes local variations in electron emission to generate image contrast. The excitation is usually produced by ultraviolet light, synchrotron radiation or X-ray sources. PEEM measures the coefficient indirectly by collecting the emitted secondary electrons generated in the electron cascade that follows the creation of the primary core hole in the absorption process. PEEM is a surface sensitive technique because the emitted electrons originate from a shallow layer. In physics, this technique is referred to as PEEM, which goes together naturally with low-energy electron diffraction (LEED), and low-energy electron microscopy (LEEM). In biology, it is called photoelectron microscopy (PEM), which fits with photoelectron spectroscopy (PES), transmission electron microscopy (TEM), and scanning electron microscopy (SEM).

X-ray spectroscopy is a general term for several spectroscopic techniques for characterization of materials by using x-ray excitation.

The term quantum efficiency (QE) may apply to incident photon to converted electron (IPCE) ratio of a photosensitive device, or it may refer to the TMR effect of a Magnetic Tunnel Junction.
Resonance Raman spectroscopy is a Raman spectroscopy technique in which the incident photon energy is close in energy to an electronic transition of a compound or material under examination. The frequency coincidence can lead to greatly enhanced intensity of the Raman scattering, which facilitates the study of chemical compounds present at low concentrations.
Electron spectroscopy refers to a group formed by techniques based on the analysis of the energies of emitted electrons such as photoelectrons and Auger electrons. This group includes X-ray photoelectron spectroscopy (XPS), which also known as Electron Spectroscopy for Chemical Analysis (ESCA), Electron energy loss spectroscopy (EELS), Ultraviolet photoelectron spectroscopy (UPS), and Auger electron spectroscopy (AES). These analytical techniques are used to identify and determine the elements and their electronic structures from the surface of a test sample. Samples can be solids, gases or liquids.
X-ray absorption near edge structure (XANES), also known as near edge X-ray absorption fine structure (NEXAFS), is a type of absorption spectroscopy that indicates the features in the X-ray absorption spectra (XAS) of condensed matter due to the photoabsorption cross section for electronic transitions from an atomic core level to final states in the energy region of 50–100 eV above the selected atomic core level ionization energy, where the wavelength of the photoelectron is larger than the interatomic distance between the absorbing atom and its first neighbour atoms.

Angle-resolved photoemission spectroscopy (ARPES) is an experimental technique used in condensed matter physics to probe the allowed energies and momenta of the electrons in a material, usually a crystalline solid. It is based on the photoelectric effect, in which an incoming photon of sufficient energy ejects an electron from the surface of a material. By directly measuring the kinetic energy and emission angle distributions of the emitted photoelectrons, the technique can map the electronic band structure and Fermi surfaces. ARPES is best suited for the study of one- or two-dimensional materials. It has been used by physicists to investigate high-temperature superconductors, graphene, topological materials, quantum well states, and materials exhibiting charge density waves.
Ultraviolet photoelectron spectroscopy (UPS) refers to the measurement of kinetic energy spectra of photoelectrons emitted by molecules which have absorbed ultraviolet photons, in order to determine molecular orbital energies in the valence region.

The Planck constant, or Planck's constant, is a fundamental physical constant denoted , and is of fundamental importance in quantum mechanics. A photon's energy is equal to its frequency multiplied by the Planck constant. Due to mass–energy equivalence, the Planck constant also relates mass to frequency.
Laser-based angle-resolved photoemission spectroscopy is a form of angle-resolved photoemission spectroscopy that uses a laser as the light source. Photoemission spectroscopy is a powerful and sensitive experimental technique to study surface physics. It is based on the photoelectric effect originally observed by Heinrich Hertz in 1887 and later explained by Albert Einstein in 1905 that when a material is shone by light, the electrons can absorb photons and escape from the material with the kinetic energy: , where is the incident photon energy, the work function of the material. Since the kinetic energy of ejected electrons are highly associated with the internal electronic structure, by analyzing the photoelectron spectroscopy one can realize the fundamental physical and chemical properties of the material, such as the type and arrangement of local bonding, electronic structure and chemical composition.
Surface-extended X-ray absorption fine structure (SEXAFS) is the surface-sensitive equivalent of the EXAFS technique. This technique involves the illumination of the sample by high-intensity X-ray beams from a synchrotron and monitoring their photoabsorption by detecting in the intensity of Auger electrons as a function of the incident photon energy. Surface sensitivity is achieved by the interpretation of data depending on the intensity of the Auger electrons instead of looking at the relative absorption of the X-rays as in the parent method, EXAFS.
Photoelectrochemical processes are processes in photoelectrochemistry; they usually involve transforming light into other forms of energy. These processes apply to photochemistry, optically pumped lasers, sensitized solar cells, luminescence, and photochromism.

Time-resolved two-photon photoelectron (2PPE) spectroscopy is a time-resolved spectroscopy technique which is used to study electronic structure and electronic excitations at surfaces. The technique utilizes femtosecond to picosecond laser pulses in order to first photoexcite an electron. After a time delay, the excited electron is photoemitted into a free electron state by a second pulse. The kinetic energy and the emission angle of the photoelectron are measured in an electron energy analyzer. To facilitate investigations on the population and relaxation pathways of the excitation, this measurement is performed at different time delays.
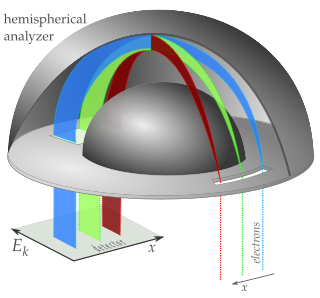
A hemispherical electron energy analyzer or hemispherical deflection analyzer is a type of electron energy spectrometer generally used for applications where high energy resolution is needed—different varieties of electron spectroscopy such as angle-resolved photoemission spectroscopy (ARPES), X-ray photoelectron spectroscopy (XPS) and Auger electron spectroscopy (AES) or in imaging applications such as photoemission electron microscopy (PEEM) and low-energy electron microscopy (LEEM).















