Related Research Articles

A myelodysplastic syndrome (MDS) is one of a group of cancers in which immature blood cells in the bone marrow do not mature, and as a result, do not develop into healthy blood cells. Early on, no symptoms typically are seen. Later, symptoms may include fatigue, shortness of breath, bleeding disorders, anemia, or frequent infections. Some types may develop into acute myeloid leukemia.
Aplastic anemia (AA) is a severe hematologic condition in which the body fails to make blood cells in sufficient numbers. Blood cells are produced in the bone marrow by stem cells that reside there. Aplastic anemia causes a deficiency of all blood cell types: red blood cells, white blood cells, and platelets.
Neutropenia is an abnormally low concentration of neutrophils in the blood. Neutrophils make up the majority of circulating white blood cells and serve as the primary defense against infections by destroying bacteria, bacterial fragments and immunoglobulin-bound viruses in the blood. People with neutropenia are more susceptible to bacterial infections and, without prompt medical attention, the condition may become life-threatening.
TAR syndrome is a rare genetic disorder that is characterized by the absence of the radius bone in the forearm and a dramatically reduced platelet count. It is associated with cardiac defects, dysmorphic features, and petechiae. It involves a 1q21 deletion with RMB8A variant on other allele.

Immune thrombocytopenic purpura (ITP), also known as idiopathic thrombocytopenic purpura or immune thrombocytopenia, is an autoimmune primary disorder of hemostasis characterized by a low platelet count in the absence of other causes. ITP often results in an increased risk of bleeding from mucosal surfaces or the skin. Depending on which age group is affected, ITP causes two distinct clinical syndromes: an acute form observed in children and a chronic form in adults. Acute ITP often follows a viral infection and is typically self-limited, while the more chronic form does not yet have a specific identified cause. Nevertheless, the pathogenesis of ITP is similar in both syndromes involving antibodies against various platelet surface antigens such as glycoproteins.
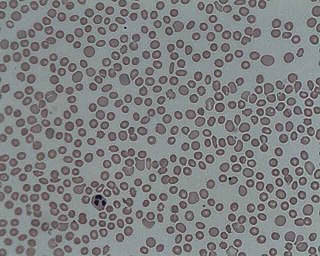
In hematology, thrombocytopenia is a condition characterized by abnormally low levels of platelets in the blood. Low levels of platelets in turn may lead to prolonged or excessive bleeding. It is the most common coagulation disorder among intensive care patients and is seen in a fifth of medical patients and a third of surgical patients.

Thrombopoietin (THPO) also known as megakaryocyte growth and development factor (MGDF) is a protein that in humans is encoded by the THPO gene.
Evans syndrome is an autoimmune disease in which an individual's immune system attacks their own red blood cells and platelets, the syndrome can include immune neutropenia. These immune cytopenias may occur simultaneously or sequentially.
Pancytopenia is a medical condition in which there is significant reduction in the number of almost all blood cells.

Acute myeloid leukemia (AML) is a cancer of the myeloid line of blood cells, characterized by the rapid growth of abnormal cells that build up in the bone marrow and blood and interfere with normal blood cell production. Symptoms may include feeling tired, shortness of breath, easy bruising and bleeding, and increased risk of infection. Occasionally, spread may occur to the brain, skin, or gums. As an acute leukemia, AML progresses rapidly, and is typically fatal within weeks or months if left untreated.
An exchange transfusion is a blood transfusion in which the patient's blood or components of it are exchanged with other blood or blood products. The patient's blood is removed and replaced by donated blood or blood components. This exchange transfusion can be performed manually or using a machine (apheresis).

Chronic myelomonocytic leukemia (CMML) is a type of leukemia, which are cancers of the blood-forming cells of the bone marrow. In adults, blood cells are formed in the bone marrow, by a process that is known as haematopoiesis. In CMML, there are increased numbers of monocytes and immature blood cells (blasts) in the peripheral blood and bone marrow, as well as abnormal looking cells (dysplasia) in at least one type of blood cell.
Hepatic veno-occlusive disease (VOD) or veno-occlusive disease with immunodeficiency is a potentially life-threatening condition in which some of the small veins in the liver are obstructed. It is a complication of high-dose chemotherapy given before a bone marrow transplant and/or excessive exposure to hepatotoxic pyrrolizidine alkaloids. It is classically marked by weight gain due to fluid retention, increased liver size, and raised levels of bilirubin in the blood. The name sinusoidal obstruction syndrome (SOS) is preferred if hepatic veno-occlusive disease happens as a result of chemotherapy or bone marrow transplantation.

Sickle cell disease (SCD), also simply called sickle cell, is a group of hemoglobin-related blood disorders that are typically inherited. The most common type is known as sickle cell anemia. Sickle cell anemia results in an abnormality in the oxygen-carrying protein haemoglobin found in red blood cells. This leads to the red blood cells adopting an abnormal sickle-like shape under certain circumstances; with this shape, they are unable to deform as they pass through capillaries, causing blockages. Problems in sickle cell disease typically begin around 5 to 6 months of age. A number of health problems may develop, such as attacks of pain in joints, anemia, swelling in the hands and feet, bacterial infections, dizziness and stroke. The probability of severe symptoms, including long-term pain, increases with age. Without treatment, people with SCD rarely reach adulthood but with good healthcare, median life expectancy is between 58 and 66 years. All the major organs are affected by sickle cell disease. The liver, heart, kidneys, gallbladder, eyes, bones, and joints also can suffer damage from the abnormal functions of the sickle cells, and their inability to flow through the small blood vessels correctly.

Blastic plasmacytoid dendritic cell neoplasm (BPDCN) is a rare hematologic malignancy. It was initially regarded as a form of lymphocyte-derived cutaneous lymphoma and alternatively named CD4+CD56+ hematodermic tumor, blastic NK cell lymphoma, and agranular CD4+ NK cell leukemia. Later, however, the disease was determined to be a malignancy of plasmacytoid dendritic cells rather than lymphocytes and therefore termed blastic plasmacytoid dendritic cell neoplasm. In 2016, the World Health Organization designated BPDCN to be in its own separate category within the myeloid class of neoplasms. It is estimated that BPDCN constitutes 0.44% of all hematological malignancies.
Red blood cells (erythrocytes) from donors contain normal hemoglobin (HbA), and transfusion of normal red blood cells into people with sickle cell disease reduces the percentage of red cells in the circulation containing the abnormal hemoglobin (HbS). Although transfusion of donor red blood cells can ameliorate and even prevent complications of sickle cell disease in certain circumstances, transfusion therapy is not universally beneficial in sickle cell disease.
GATA2 deficiency is a grouping of several disorders caused by common defect, namely, familial or sporadic inactivating mutations in one of the two parental GATA2 genes. Being the gene haploinsufficient, mutations that cause a reduction in the cellular levels of the gene's product, GATA2, are autosomal dominant. The GATA2 protein is a transcription factor critical for the embryonic development, maintenance, and functionality of blood-forming, lymphatic-forming, and other tissue-forming stem cells. In consequence of these mutations, cellular levels of GATA2 are deficient and individuals develop over time hematological, immunological, lymphatic, or other presentations that may begin as apparently benign abnormalities but commonly progress to severe organ failure, opportunistic infections, virus infection-induced cancers, the myelodysplastic syndrome, and/or leukemia. GATA2 deficiency is a life-threatening and precancerous condition.

Swee Lay Thein is a Malaysian haematologist and physician-scientist who is Senior Investigator at the National Institutes of Health. She works on the pathophysiology of haemoglobin disorders including sickle cell disease and thalassemia.

Marina Cavazzana is a professor of Paediatric Immunology at the Necker-Enfants Malades Hospital and the Imagine Institute, as well as an academic at Paris Descartes University. She was awarded the Irène Joliot-Curie Prize in 2012 and elected to the National Academy of Medicine in 2019.
Louise E. Purton is an Australian biologist who is Professor of Medicine and head of the Stem Cell Regulation Laboratory at St. Vincent's Institute of Medical Research in Melbourne. Her research considers the stem cells responsible for the production of blood cells and the regulations of haematopoietic diseases. She was awarded the International Society for Experimental Hematology McCulloch & Till Award in 2022. She has experienced profound bilateral hearing loss since the age of three and has been recognised for her work supporting Equity and Diversity, particularly amongst women and people with disability, and is a member of the AAMRI Gender, Equity and Diversity and Inclusion group GEDI.
References
- ↑ "Irene Roberts". www.imm.ox.ac.uk. Retrieved 2024-10-10.
- 1 2 Roberts I, Hann I (November 2020). "The genesis of paediatric haematology in the UK". British Journal of Haematology. 191 (4): 593–603. doi:10.1111/bjh.17163. PMID 33190251.
- ↑ Roberts, Irene. "The Road to Beating Childhood Leukaemia". Blood Cancer UK. Retrieved 10 October 2024.
- ↑ Mobley, Immy (October 4, 2021). "Understanding prenatal haematopoiesis in bone marrow". Front Line Genomics. Retrieved 10 October 2024.
- ↑ Jardine L, Webb S, Goh I, Quiroga Londoño M, Reynolds G, Mather M, Olabi B, Stephenson E, Botting RA, Roberts I, Teichmann SA, Haniffa M (October 2021). "Blood and immune development in human fetal bone marrow and Down syndrome". Nature. 598 (7880): 327–331. Bibcode:2021Natur.598..327J. doi:10.1038/s41586-021-03929-x. PMC 7612688 . PMID 34588693.
- ↑ "Irene A. Roberts". Scopus. Retrieved October 9, 2024.
- ↑ "Irene Roberts delivers Ham-Wasserman Lecture". University of Oxford Department of Paediatrics. January 13, 2023. Retrieved 10 October 2024.
- ↑ Roberts I (December 2022). "Leukemogenesis in infants and young children with trisomy 21". Hematology. American Society of Hematology. Education Program. 2022 (1): 1–8. doi:10.1182/hematology.2022000395. PMC 9820574 . PMID 36485097.
- ↑ Iskander D, Karadimitris A, Roberts I (July 2024). "Harnessing Single-Cell Technologies in the Search for New Therapies for Diamond-Blackfan Anemia Syndrome". Experimental Hematology. 135: 104235. doi: 10.1016/j.exphem.2024.104235 . PMID 38740323.
- ↑ Roberts I, Vyas P (July 2021). "Sowing the seeds of leukemia before birth". Science. 373 (6551): 155–156. Bibcode:2021Sci...373..155R. doi:10.1126/science.abj3957. PMID 34244395.
- ↑ Popescu DM, Botting RA, Stephenson E, Green K, Roberts I, Göttgens B, Behjati S, Laurenti E, Teichmann SA, Haniffa M (October 2019). "Decoding human fetal liver haematopoiesis". Nature. 574 (7778): 365–371. Bibcode:2019Natur.574..365P. doi:10.1038/s41586-019-1652-y. PMC 6861135 . PMID 31597962.
- ↑ Roberts I, Fordham NJ, Rao A, Bain BJ (July 2018). "Neonatal leukaemia". British Journal of Haematology. 182 (2): 170–184. doi:10.1111/bjh.15246. PMID 29806701.
- ↑ Roberts I, de la Fuente J (November 2016). "Sickle cell disease: the price of cure". Blood. 128 (21): 2486–2488. doi:10.1182/blood-2016-10-740969. PMID 27884835.
- ↑ Roberts I, Izraeli S (December 2014). "Haematopoietic development and leukaemia in Down syndrome". British Journal of Haematology. 167 (5): 587–99. doi:10.1111/bjh.13096. PMID 25155832.
- ↑ Chakravorty S, Roberts I (January 2012). "How I manage neonatal thrombocytopenia". British Journal of Haematology. 156 (2): 155–62. doi:10.1111/j.1365-2141.2011.08892.x. PMID 21950766.
- ↑ Roberts IA (August 2008). "The changing face of haemolytic disease of the newborn". Early Human Development. 84 (8): 515–23. doi:10.1016/j.earlhumdev.2008.06.005. PMID 18621490.
- ↑ Roberts IA, Murray NA (March 2006). "Neonatal thrombocytopenia". Current Hematology Reports. 5 (1): 55–63. PMID 16537047.
- ↑ Halsey C, Roberts IA (January 2003). "The role of hydroxyurea in sickle cell disease". British Journal of Haematology. 120 (2): 177–86. doi:10.1046/j.1365-2141.2003.03849.x. PMID 12542474.
- ↑ "Meet Irene Roberts, our September volunteer of the month". European Hematology Association. September 19, 2019. Retrieved 10 October 2024.