
Chlorophyll is any of several related green pigments found in cyanobacteria and the chloroplasts of algae and plants. Its name is derived from the Greek words χλωρός, chloros ("green") and φύλλον, phyllon ("leaf"). Chlorophyll is essential in photosynthesis, allowing plants to absorb energy from light.
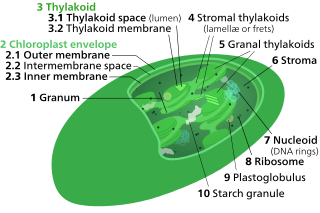
A thylakoid is a membrane-bound compartment inside chloroplasts and cyanobacteria. They are the site of the light-dependent reactions of photosynthesis. Thylakoids consist of a thylakoid membrane surrounding a thylakoid lumen. Chloroplast thylakoids frequently form stacks of disks referred to as grana. Grana are connected by intergranal or stroma thylakoids, which join granum stacks together as a single functional compartment.

Photosystems are functional and structural units of protein complexes involved in photosynthesis that together carry out the primary photochemistry of photosynthesis: the absorption of light and the transfer of energy and electrons. Photosystems are found in the thylakoid membranes of plants, algae and cyanobacteria. They are located in the chloroplasts of plants and algae, and in the cytoplasmic membrane of photosynthetic bacteria. There are two kinds of photosystems: II and I.

Photosystem II is the first protein complex in the light-dependent reactions of oxygenic photosynthesis. It is located in the thylakoid membrane of plants, algae, and cyanobacteria. Within the photosystem, enzymes capture photons of light to energize electrons that are then transferred through a variety of coenzymes and cofactors to reduce plastoquinone to plastoquinol. The energized electrons are replaced by oxidizing water to form hydrogen ions and molecular oxygen.

Photosystem I is the second photosystem in the photosynthetic light reactions of algae, plants, and some bacteria. Photosystem I is an integral membrane protein complex that uses light energy to produce the high energy carriers ATP and NADPH. PSI comprises more than 110 cofactors, significantly more than photosystem II.
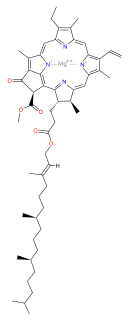
Chlorophyll a is a specific form of chlorophyll used in oxygenic photosynthesis. It absorbs most energy from wavelengths of violet-blue and orange-red light. It also reflects green-yellow light, and as such contributes to the observed green color of most plants. This photosynthetic pigment is essential for photosynthesis in eukaryotes, cyanobacteria and prochlorophytes because of its role as primary electron donor in the electron transport chain. Chlorophyll a also transfers resonance energy in the antenna complex, ending in the reaction center where specific chlorophylls P680 and P700 are located.
Accessory pigments are light-absorbing compounds, found in photosynthetic organisms, that work in conjunction with chlorophyll a. They include other forms of this pigment, such as chlorophyll b in green algal and higher plant antennae, while other algae may contain chlorophyll c or d. In addition, there are many non-chlorophyll accessory pigments, such as carotenoids or phycobiliproteins, which also absorb light and transfer that light energy to photosystem chlorophyll. Some of these accessory pigments, in particular the carotenoids, also serve to absorb and dissipate excess light energy, or work as antioxidants. The large, physically associated group of chlorophylls and other accessory pigments is sometimes referred to as a pigment bed.
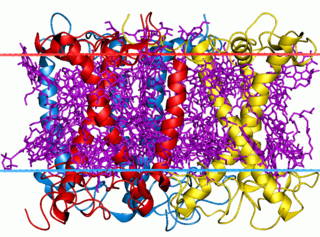
The light-harvesting complex is an array of protein and chlorophyll molecules embedded in the thylakoid membrane of plants, which transfer light energy to one chlorophyll a molecule at the reaction center of a photosystem.

A light-harvesting complex has a complex of subunit proteins that may be part of a larger supercomplex of a photosystem, the functional unit in photosynthesis. It is used by plants and photosynthetic bacteria to collect more of the incoming light than would be captured by the photosynthetic reaction center alone. Light-harvesting complexes are found in a wide variety among the different photosynthetic species. The complexes consist of proteins and photosynthetic pigments and surround a photosynthetic reaction center to focus energy, attained from photons absorbed by the pigment, toward the reaction center using Förster resonance energy transfer.
Photoprotection is the biochemical process that helps organisms cope with molecular damage caused by sunlight. Plants and other oxygenic phototrophs have developed a suite of photoprotective mechanisms to prevent photoinhibition and oxidative stress caused by excess or fluctuating light conditions. Humans and other animals have also developed photoprotective mechanisms to avoid UV photodamage to the skin, prevent DNA damage, and minimize the downstream effects of oxidative stress.

Biohydrogen is H2 that is produced biologically. Interest is high in this technology because H2 is a clean fuel and can be readily produced from certain kinds of biomass. Many challenges characterize this technology, including those intrinsic to H2, such as storage and transportation of a noncondensible gas. Hydrogen producing organisms are poisoned by O2. Yields of H2 are often low.

Photoinhibition is light-induced reduction in the photosynthetic capacity of a plant, alga, or cyanobacterium. Photosystem II (PSII) is more sensitive to light than the rest of the photosynthetic machinery, and most researchers define the term as light-induced damage to PSII. In living organisms, photoinhibited PSII centres are continuously repaired via degradation and synthesis of the D1 protein of the photosynthetic reaction center of PSII. Photoinhibition is also used in a wider sense, as dynamic photoinhibition, to describe all reactions that decrease the efficiency of photosynthesis when plants are exposed to light.
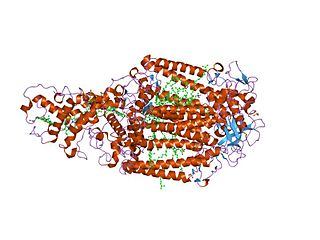
Photosynthetic reaction centre proteins are main protein components of photosynthetic reaction centres of bacteria and plants.

Photosystem II light-harvesting proteins are the intrinsic transmembrane proteins CP43 (PsbC) and CP47 (PsbB) occurring in the reaction centre of photosystem II. These polypeptides bind to chlorophyll a and beta-carotene and pass the excitation energy on to the reaction centre. This family also includes the iron-stress induced chlorophyll-binding protein CP43' (IsiA,CP43'), which evolved in cyanobacteria from a PSII protein to cope with light limitations and stress conditions.
Non-photochemical quenching (NPQ) is a mechanism employed by plants and algae to protect themselves from the adverse effects of high light intensity. It involves the quenching of singlet excited state chlorophylls (Chl) via enhanced internal conversion to the ground state, thus harmlessly dissipating excess excitation energy as heat through molecular vibrations. NPQ occurs in almost all photosynthetic eukaryotes, and helps to regulate and protect photosynthesis in environments where light energy absorption exceeds the capacity for light utilization in photosynthesis.

In photosynthesis, the light-dependent reactions take place on the thylakoid membranes. The inside of the thylakoid membrane is called the lumen, and outside the thylakoid membrane is the stroma, where the light-independent reactions take place. The thylakoid membrane contains some integral membrane protein complexes that catalyze the light reactions. There are four major protein complexes in the thylakoid membrane: Photosystem II (PSII), Cytochrome b6f complex, Photosystem I (PSI), and ATP synthase. These four complexes work together to ultimately create the products ATP and NADPH.

The psaA RNA motif describes a class of RNAs with a common secondary structure. psaA RNAs are exclusively found in locations that presumably correspond to the 5' untranslated regions of operons formed of psaA and psaB genes. For this reason, it was hypothesized that psaA RNAs function as cis-regulatory elements of these genes. The psaAB genes encode proteins that form subunits in the photosystem I structure used for photosynthesis. psaA RNAs have been detected only in cyanobacteria, which is consistent with their association with photosynthesis.
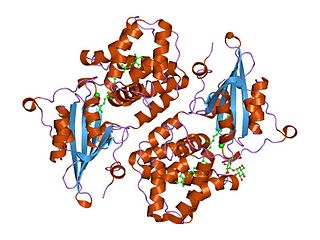
In molecular biology the orange carotenoid N-terminal domain is a protein domain found predominantly at the N-terminus of the Orange carotenoid protein (OCP), and is involved in non-covalent binding of a carotenoid chromophore. It is unique for being present in soluble proteins, whereas the vast majority of domains capable of binding carotenoids are intrinsic membrane proteins. Thus far, it has exclusively been found in cyanobacteria, among which it is widespread. The domain also exists on its own, in uncharacterized cyanobacterial proteins referred to as "Red Carotenoid Protein" (RCP). The domain adopts an alpha-helical structure consisting of two four-helix bundles.
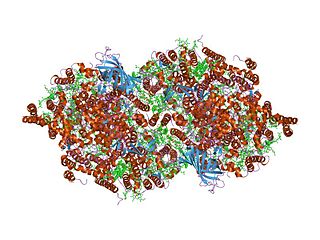
In molecular biology, the PsbZ (Ycf9) is a protein domain, which is low in molecular weight. It is a transmembrane protein and therefore is located in the thylakoid membrane of chloroplasts in cyanobacteria and plants. More specifically, it is located in Photosystem II (PSII) and in the light-harvesting complex II (LHCII). Ycf9 acts as a structural linker, that stabilises the PSII-LHCII supercomplexes. Moreover, the supercomplex fails to form in PsbZ-deficient mutants, providing further evidence to suggest Ycf9's role as a structural linker. This may be caused by a marked decrease in two LHCII antenna proteins, CP26 and CP29, found in PsbZ-deficient mutants, which result in structural changes, as well as functional modifications in PSII.

Orange carotenoid protein (OCP) is a water-soluble protein which plays a role in photoprotection in diverse cyanobacteria. It is the only photoactive protein known to use a carotenoid as the photoresponsive chromophore. The protein consists of two domains, with a single keto-carotenoid molecule non-covalently bound between the two domains. It is a very efficient quencher of excitation energy absorbed by the primary light-harvesting antenna complexes of cyanobacteria, the phycobilisomes. The quenching is induced by blue-green light. It is also capable of preventing oxidative damage by directly scavenging singlet oxygen (1O2).














