
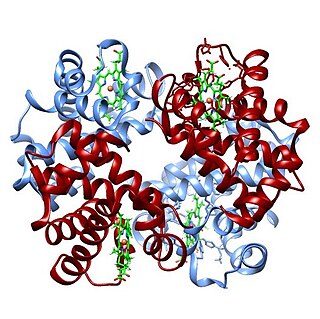
Hemoglobin,, abbreviated Hb or Hgb, is the iron-containing oxygen-transport metalloprotein in red blood cells (erythrocytes) of almost all vertebrates as well as the tissues of some invertebrates. Hemoglobin in blood carries oxygen from the respiratory organs to the rest of the body. There it releases the oxygen to permit aerobic respiration to provide energy to power functions of an organism in the process called metabolism. A healthy individual human has 12 to 20 grams of hemoglobin in every 100 mL of blood.

Clinical chemistry is the area of chemistry that is generally concerned with analysis of bodily fluids for diagnostic and therapeutic purposes. It is an applied form of biochemistry.

Iron deficiency, or sideropenia, is the state in which a body lacks enough iron to supply its needs. Iron is present in all cells in the human body and has several vital functions, such as carrying oxygen to the tissues from the lungs as a key component of the hemoglobin protein, acting as a transport medium for electrons within the cells in the form of cytochromes, and facilitating oxygen enzyme reactions in various tissues. Too little iron can interfere with these vital functions and lead to morbidity and death.
Reference ranges for blood tests are sets of values used by a health professional to interpret a set of medical test results from blood samples. Reference ranges for blood tests are studied within the field of clinical chemistry, the area of pathology that is generally concerned with analysis of bodily fluids.
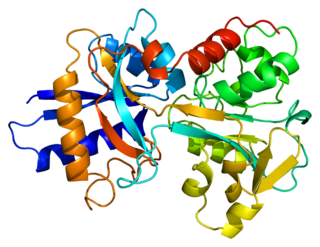
Transferrins are glycoproteins found in vertebrates which bind to and consequently mediate the transport of iron (Fe) through blood plasma. They are produced in the liver and contain binding sites for two Fe3+ ions. Human transferrin is encoded by the TF gene and produced as a 76 kDa glycoprotein.

Hereditary spherocytosis (HS) is a congenital hemolytic disorder, wherein a genetic mutation coding for a structural membrane protein phenotype leads to a spherical shaping of erythrocytic cellular morphology. As erythrocytes are sphere-shaped (spherocytosis), rather than the normal biconcave disk-shaped, their morphology interferes with these cells' abilities to be flexible during circulation throughout the entirety of the body - arteries, arterioles, capillaries, venules, veins, and organs. This difference in shape also makes the red blood cells more prone to rupture under osmotic and/or mechanical stress. Cells with these dysfunctional proteins are degraded in the spleen, which leads to a shortage of erythrocytes resulting in hemolytic anemia.

Iron-deficiency anemia is anemia caused by a lack of iron. Anemia is defined as a decrease in the number of red blood cells or the amount of hemoglobin in the blood. When onset is slow, symptoms are often vague such as feeling tired, weak, short of breath, or having decreased ability to exercise. Anemia that comes on quickly often has more severe symptoms, including: confusion, feeling like one is going to pass out or increased thirst. Anemia is typically significant before a person becomes noticeably pale. Children with iron deficiency anemia may have problems with growth and development. There may be additional symptoms depending on the underlying cause.

The mean corpuscular hemoglobin concentration (MCHC) is a measure of the concentration of hemoglobin in a given volume of packed red blood cell.

The mean corpuscular hemoglobin, or "mean cell hemoglobin" (MCH), is the average mass of hemoglobin (Hb) per red blood cell (RBC) in a sample of blood. It is reported as part of a standard complete blood count. MCH value is diminished in hypochromic anemias.
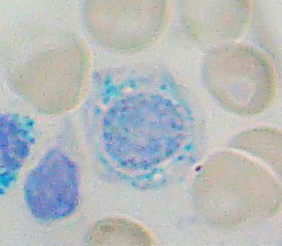
Sideroblastic anemia, or sideroachrestic anemia, is a form of anemia in which the bone marrow produces ringed sideroblasts rather than healthy red blood cells (erythrocytes). In sideroblastic anemia, the body has iron available but cannot incorporate it into hemoglobin, which red blood cells need in order to transport oxygen efficiently. The disorder may be caused either by a genetic disorder or indirectly as part of myelodysplastic syndrome, which can develop into hematological malignancies.
Serum iron is a medical laboratory test that measures the amount of circulating iron that is bound to transferrin (90%) and serum ferritin (10%). Clinicians order this laboratory test when they are concerned about iron deficiency, which can cause anemia and other problems. 65% of the iron in the body is bound up in hemoglobin molecules in red blood cells. About 4% is bound up in myoglobin molecules. Around 30% of the iron in the body is stored as ferritin or hemosiderin in the spleen, the bone marrow and the liver. Small amounts of iron can be found in other molecules in cells throughout the body. None of this iron is directly accessible by testing the serum.}

Total iron-binding capacity (TIBC) or sometimes transferrin iron-binding capacity is a medical laboratory test that measures the blood's capacity to bind iron with transferrin. Transferrin can bind two atoms of ferric iron (Fe3+) with high affinity. It means that transferrin has the capacity to transport approximately from 1.40 to 1.49 mg of iron per gram of transferrin present in the blood.
Transferrin saturation (TS), measured as a percentage, is a medical laboratory value. It is the value of serum iron divided by the total iron-binding capacity of the available transferrin, the main protein that binds iron in the blood, this value tells a clinician how much serum iron is bound. For instance, a value of 15% means that 15% of iron-binding sites of transferrin are being occupied by iron. The three results are usually reported together. A low transferrin saturation is a common indicator of iron deficiency anemia whereas a high transferrin saturation may indicate iron overload or hemochromatosis. Transferrin saturation is also called transferrin saturation index (TSI) or transferrin saturation percentage (TS%)

African iron overload, also known as, is an iron overload disorder first observed among people of African descent in Southern Africa and Central Africa. Dietary iron overload is the consumption of large amount of home-brewed beer with high amount of iron content in it. Preparing beer in iron pots or drums results in high iron content. The iron content in home-brewed beer is around 46–82 mg/l compared to 0.5 mg/l in commercial beer. Dietary overload was prevalent in both the rural and urban Black African population, with the introduction of commercial beer in urban areas, the condition has decreased. However, the condition is still common in rural areas. Until recently, studies have shown that genetics might play a role in this disorder. Combination of excess iron and functional changes in ferroportin seems to be the probable cause. This disorder can be treated with phlebotomy therapy or therapy.
The oxygen–hemoglobin dissociation curve, also called the oxyhemoglobin dissociation curve or oxygen dissociation curve (ODC), is a curve that plots the proportion of hemoglobin in its saturated (oxygen-laden) form on the vertical axis against the prevailing oxygen tension on the horizontal axis. This curve is an important tool for understanding how our blood carries and releases oxygen. Specifically, the oxyhemoglobin dissociation curve relates oxygen saturation (SO2) and partial pressure of oxygen in the blood (PO2), and is determined by what is called "hemoglobin affinity for oxygen"; that is, how readily hemoglobin acquires and releases oxygen molecules into the fluid that surrounds it.

Hypochromic anemia is a generic term for any type of anemia in which the red blood cells are paler than normal. A normal red blood cell has a biconcave disk shape and will have an area of pallor in its center when viewed microscopically. In hypochromic cells, this area of central pallor is increased. This decrease in redness is due to a disproportionate reduction of red cell hemoglobin in proportion to the volume of the cell. Clinically the color can be evaluated by the mean corpuscular hemoglobin (MCH) or mean corpuscular hemoglobin concentration (MCHC). The MCHC is considered the better parameter of the two as it adjusts for effect the size of the cell has on its amount of hemoglobin. Hypochromia is clinically defined as below the normal MCH reference range of 27–33 picograms/cell in adults or below the normal MCHC reference range of 33–36 g/dL in adults.
Iron-binding proteins are carrier proteins and metalloproteins that are important in iron metabolism and the immune response. Iron is required for life.
Red blood cell indices are blood tests that provide information about the hemoglobin content and size of red blood cells. Abnormal values indicate the presence of anemia and which type of anemia it is.
Latent iron deficiency (LID), also called iron-deficient erythropoiesis, is a medical condition in which there is evidence of iron deficiency without anemia. It is important to assess this condition because individuals with latent iron deficiency may develop iron-deficiency anemia. Additionally, there is some evidence of a decrease in vitality and an increase in fatigue among individuals with LID.
Anemia is a condition in which blood has a lower-than-normal amount of red blood cells or hemoglobin. Anemia in pregnancy is a decrease in the total red blood cells (RBCs) or hemoglobin in the blood during pregnancy. Anemia is an extremely common condition in pregnancy world-wide, conferring a number of health risks to mother and child. While anemia in pregnancy may be pathologic, in normal pregnancies, the increase in RBC mass is smaller than the increase in plasma volume, leading to a mild decrease in hemoglobin concentration referred to as physiologic(or dilutional) anemia. Maternal signs and symptoms are usually non-specific, but can include: fatigue, pallor, dyspnea, palpitations, and dizziness. There are numerous well-known maternal consequences of anemia including: maternal cardiovascular strain, reduced physical and mental performance, reduced peripartum blood reserves, increased risk for peripartum blood product transfusion, and increased risk for maternal mortality.









