Mechanism
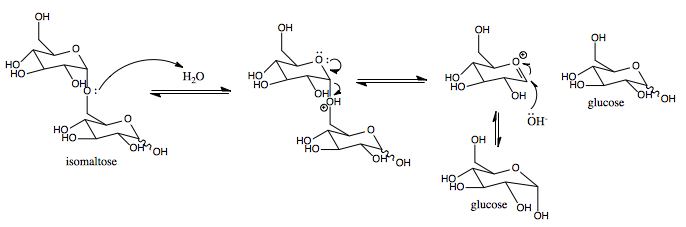
This enzyme catalyses the following chemical reaction
- Hydrolysis of (1->6)-alpha-D-glucosidic linkages in some oligosaccharides produced from starch and glycogen by enzyme EC 3.2.1.1.
Hydrolysis uses water to cleave chemical bonds. Sucrase-isomaltase’s mechanism results in a net retention of configuration at the anomeric center. [1]