Vapor pressure of liquid
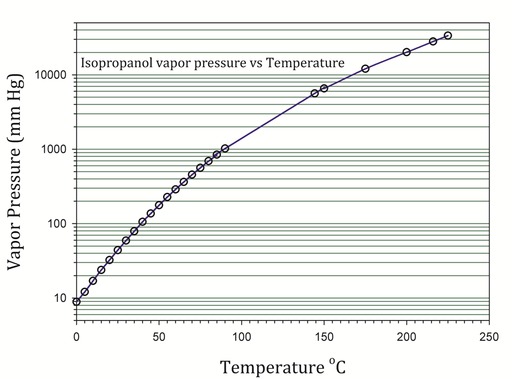
| P in mm Hg | 1 | 10 | 40 | 100 | 400 | 760 | 1520 | 3800 | 7600 | 15200 | 30400 | 45600 | |
| T in °C | −26.1 | 2.4 | 23.8 | 39.5 | 67.8 | 82.5 | 101.3 | 130.2 | 155.7 | 186.0 | 220.2 | — | |
Table data obtained from CRC Handbook of Chemistry and Physics 44th ed.
This page provides supplementary chemical data on isopropanol.
The handling of this chemical may incur notable safety precautions. It is highly recommend that you seek the Material Safety Datasheet (MSDS) for this chemical from a reliable source such as eChemPortal, and follow its directions.
| Structure and properties | |
|---|---|
| Index of refraction, nD | 1.3776 at 20°C |
| Abbe number | ? |
| Dielectric constant, εr | 18.23 ε0 at 25 °C |
| Bond strength | ? |
| Bond length | ? |
| Bond angle | ? |
| Magnetic susceptibility | ? |
| Surface tension | 21.7 dyn/cm at 20°C |
| Viscosity [1] | 4.5646 mPa·s at 0°C 2.3703 mPa·s at 20°C 1.3311 mPa·s at 40°C |
| Phase behavior | |
|---|---|
| Triple point | 184.9 K (−88.2 °C), ? Pa |
| Critical point | 508.7 K (235.6 °C), 5370 kPa |
| Std enthalpy change of fusion, ΔfusH | 5.28 kJ/mol |
| Std entropy change of fusion, ΔfusS | 28.6 J/(mol·K) |
| Std enthalpy change of vaporization, ΔvapH | 44.0 kJ/mol |
| Std entropy change of vaporization, ΔvapS | 124 J/(mol·K) |
| Solid properties | |
| Std enthalpy change of formation, ΔfH | ? kJ/mol |
| Standard molar entropy, S | ? J/(mol K) |
| Heat capacity, cp | 0.212 J/(mol K) at −200°C |
| Liquid properties | |
| Std enthalpy change of formation, ΔfH | −318.2 kJ/mol |
| Standard molar entropy, S | 180 J/(mol K) |
| Heat capacity, cp | 2.68 J/(gK) at 20°C-25°C |
| Gas properties | |
| Std enthalpy change of formation, ΔfH | −261.1 kJ/mol |
| Standard molar entropy, S | 333 J/(mol K) |
| Heat capacity, cp | 1.54 J/(gK) at 25°C |
| P in mm Hg | 1 | 10 | 40 | 100 | 400 | 760 | 1520 | 3800 | 7600 | 15200 | 30400 | 45600 | |
| T in °C | −26.1 | 2.4 | 23.8 | 39.5 | 67.8 | 82.5 | 101.3 | 130.2 | 155.7 | 186.0 | 220.2 | — | |
Table data obtained from CRC Handbook of Chemistry and Physics 44th ed.

|
|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
See also
| UV-Vis | |
|---|---|
| λmax | 205 nm |
| Extinction coefficient, ε | 100.2 m2/mol |
| IR | |
| Major absorption bands | 3334, 2970, 1466, 1378, 1160, 1128, 951, 817, 639 cm−1 |
| NMR | |
| Proton NMR | |
| Carbon-13 NMR | |
| Other NMR data | |
| MS | |
| Masses of main fragments | m/z (% of relative intensity): 45 (100), 43 (19.1), 27 (16.8), 29 (12.5), 19 (9.9), 15 (9.5), 41 (8.2), 31 (6.8), 39 (6.6), 42 (4.4) |

Distillation, or classical distillation, is the process of separating the components or substances from a liquid mixture by using selective boiling and condensation. Dry distillation is the heating of solid materials to produce gaseous products. Dry distillation may involve chemical changes such as destructive distillation or cracking and is not discussed under this article. Distillation may result in essentially complete separation, or it may be a partial separation that increases the concentration of selected components in the mixture. In either case, the process exploits differences in the relative volatility of the mixture's components. In industrial applications, distillation is a unit operation of practically universal importance, but it is a physical separation process, not a chemical reaction.

Vapor pressure or equilibrium vapor pressure is defined as the pressure exerted by a vapor in thermodynamic equilibrium with its condensed phases at a given temperature in a closed system. The equilibrium vapor pressure is an indication of a liquid's evaporation rate. It relates to the tendency of particles to escape from the liquid. A substance with a high vapor pressure at normal temperatures is often referred to as volatile. The pressure exhibited by vapor present above a liquid surface is known as vapor pressure. As the temperature of a liquid increases, the kinetic energy of its molecules also increases. As the kinetic energy of the molecules increases, the number of molecules transitioning into a vapor also increases, thereby increasing the vapor pressure.

Freezing-point depression is a drop in the temperature at which a substance freezes, caused when a smaller amount of another, non-volatile substance is added. Examples include adding salt into water, alcohol in water, ethylene or propylene glycol in water, adding copper to molten silver, or the mixing of two solids such as impurities into a finely powdered drug.
This page provides supplementary chemical data on acetic acid.
This page provides supplementary data to the article properties of water.
This page provides supplementary chemical data on methanol.
This page provides supplementary chemical data on carbon dioxide.
This page provides supplementary chemical data on formic acid.
This page provides supplementary chemical data on ammonia.
This page provides supplementary chemical data on benzene.
This page contains tables of azeotrope data for various binary and ternary mixtures of solvents. The data include the composition of a mixture by weight, the boiling point (b.p.) of a component, the boiling point of a mixture, and the specific gravity of the mixture. Boiling points are reported at a pressure of 760 mm Hg unless otherwise stated. Where the mixture separates into layers, values are shown for upper (U) and lower (L) layers.
This page provides supplementary chemical data on glycerol.
This page provides supplementary chemical data on ethylene glycol.
This page provides supplementary chemical data on acetonitrile.
This page provides supplementary chemical data on carbon tetrachloride.
This page provides supplementary chemical data on 1-Propanol (n-propanol).
This page provides supplementary chemical data on cyclohexane.
This page provides supplementary chemical data on dimethyl sulfoxide.
This page provides supplementary chemical data on m-Xylene.
This page provides supplementary chemical data on tetrachloroethylene.