Related Research Articles
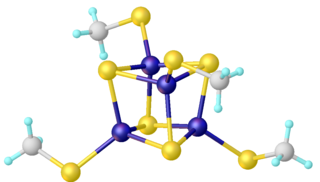
Iron–sulfur clusters are molecular ensembles of iron and sulfide. They are most often discussed in the context of the biological role for iron–sulfur proteins, which are pervasive. Many Fe–S clusters are known in the area of organometallic chemistry and as precursors to synthetic analogues of the biological clusters.

Photochemistry is the branch of chemistry concerned with the chemical effects of light. Generally, this term is used to describe a chemical reaction caused by absorption of ultraviolet, visible light (400–750 nm) or infrared radiation (750–2500 nm).
A charge-transfer complex or electron-donor-acceptor complex is an association of two or more molecules, or of different parts of one large molecule, in which a fraction of electronic charge is transferred between the molecular entities. The resulting electrostatic attraction provides a stabilizing force for the molecular complex. The source molecule from which the charge is transferred is called the electron donor and the receiving species is called the electron acceptor.

Steric effects are nonbonding interactions that influence the shape (conformation) and reactivity of ions and molecules. Steric effects complement electronic effects, which usually dictate shape and reactivity. Steric effects result from repulsive forces between overlapping electron clouds. Steric effects are widely exploited in applied and academic chemistry.
Electron transfer (ET) occurs when an electron relocates from an atom or molecule to another such chemical entity. ET is a mechanistic description of a redox reaction, wherein the oxidation state of reactant and product changes. Electron transfer is ionic bonding.

In coordination chemistry, metal ammine complexes are metal complexes containing at least one ammonia (NH3) ligand. "Ammine" is spelled this way due to historical reasons; in contrast, alkyl or aryl bearing ligands are spelt with a single "m". Almost all metal ions bind ammonia as a ligand, but the most prevalent examples of ammine complexes are for Cr(III), Co(III), Ni(II), Cu(II) as well as several platinum group metals.

Photochromism is the reversible transformation of a chemical species between two forms by the absorption of electromagnetic radiation (photoisomerization), where the two forms have different absorption spectra. In plain language, this can be described as a reversible change of colour upon exposure to light.
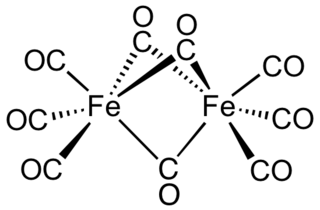
Diiron nonacarbonyl is an inorganic compound with the formula Fe2(CO)9. This metal carbonyl is an important reagent in organometallic chemistry and of occasional use in organic synthesis. It is a more reactive source of Fe(0) than Fe(CO)5 and less dangerous to handle because it is nonvolatile. This micaceous orange solid is virtually insoluble in all common solvents.

Harry Barkus Gray is the Arnold O. Beckman Professor of Chemistry at California Institute of Technology.

Daniel George Nocera is an American chemist, currently the Patterson Rockwood Professor of Energy in the Department of Chemistry and Chemical Biology at Harvard University. He is a member of the National Academy of Sciences and the American Academy of Arts and Sciences. In 2006 he was described as a "major force in the field of inorganic photochemistry and photophysics". Time magazine included him in its 2009 list of the 100 most influential people.
Adrian Ponce is the manager for the Higher Education outreach group and former Deputy Section Manager for the Planetary Science and Life Detection Section at NASA's Jet Propulsion Laboratory, and is also a visiting faculty member at Caltech. He received a BS in Chemistry from Michigan State University, and then went on to obtain a PhD in Chemistry from Caltech in 2000.

Howard E. Zimmerman aka Z was a professor of chemistry at the University of Wisconsin–Madison. He was elected to the National Academy of Sciences in 1980 and the recipient of the 1986 American Institute of Chemists Chemical Pioneer Award.
Metal aquo complexes are coordination compounds containing metal ions with only water as a ligand. These complexes are the predominant species in aqueous solutions of many metal salts, such as metal nitrates, sulfates, and perchlorates. They have the general stoichiometry [M(H2O)n]z+. Their behavior underpins many aspects of environmental, biological, and industrial chemistry. This article focuses on complexes where water is the only ligand ("homoleptic aquo complexes"), but of course many complexes are known to consist of a mix of aquo and other ligands.
Some chemical reactions take place by the action of light. These are called, "photochemical reactions", or "photolysis". Mechanistic organic photochemistry is the aspect of organic photochemistry which seeks to explain the mechanisms of organic photochemical reactions. The absorption of ultraviolet light by organic molecules often leads to reactions. In the earliest days, sunlight was employed, while in more modern times ultraviolet lamps are employed. Organic photochemistry has proven to be a very useful synthetic tool. Complex organic products can be obtained simply. Over the last century and earlier an immense number of photochemical reactions have been uncovered. In modern times the field is quite well understood and is used in organic synthesis and industrially. The utility of organic photochemistry has arisen only by virtue of the available mechanistic treatment; reactions which appear unlikely in ground-state understanding become understandable and accessible in terms of electronic excited-state consideration.

Pentaamine(nitrogen)ruthenium(II) chloride is an inorganic compound with the formula [Ru(NH3)5(N2)]Cl2. It is a nearly white solid, but its solutions are yellow. The cationic complex is of historic significance as the first compound with N2 bound to a metal center. [Ru(NH3)5(N2)]2+ adopts an octahedral structure with C4v symmetry.
John Isaiah Brauman is an American chemist.

Hiroshi Nishihara, born 21 March 1955, is a Japanese chemist and Professor of Chemistry at The University of Tokyo in Japan. Currently heading the department of Chemistry and Inorganic Chemistry Laboratory in The University of Tokyo, he is a distinguished professor, researcher and pioneer in the field of synthesis and electrochemistry of conductive metal complex polymers.

Jillian Lee Dempsey is an American inorganic chemist at the University of North Carolina at Chapel Hill. Currently, her work focuses on proton-coupled electron transfer, charge transfer events, and quantum dots. She is the recipient of numerous awards for rising stars of chemistry, including most recently a 2016 Alfred P. Sloan Research Fellowship and a 2016 Air Force's Young Investigator Research Program (YIP). Prior to working at the University of North Carolina at Chapel Hill, Dempsey was a postdoctoral researcher in the laboratory of Daniel R. Gamelin at the University of Washington.
Lisa McElwee-White is currently the Colonel Allen R. and Margaret G. Crow Professor of Chemistry at the University of Florida.
David N. Beratan is the R.J. Reynolds Professor of Chemistry at Duke University. He has secondary appointments in the departments of Physics and Biochemistry. He is the Director of the Center for Synthesizing Quantum Coherence, a NSF Phase I Center for Chemical Innovation.
References
- ↑ Electron-Transfer Kinetics of Pentammineruthenium(III)histidine-33-ferricytochrome c. Measurement of the Rate of Intramolecular Electron Transfer between Redox Centers Separated by 15 Å in a Protein Winkler, J. R.; Nocera, D. G.; Yocom, K. M.; Bordignon, E.; Gray, H. B. J. Am. Chem. Soc. 1982, 104, 5798-5800
- ↑ Characterization and Reactions of Osmium(IV) Ammines Buhr, J. D.; Winkler, J. R.; Taube, H. Inorg. Chem. 1980, 19, 2416-2425
- ↑ Emission Spectroscopic Properties of Dioxorhenium(V) Complexes in Crystals and Solutions Winkler, J. R.; Gray, H. B. J. Am. Chem. Soc. 1983, 105, 1373-1374.
- ↑ On the Role of the High-Spin State in the Water Exchange Reaction of Hexaaquocobalt(III) Winkler, J. R.; Rice, S. F.; Gray, H. Comments Inorg. Chem. 1981, 1, 47-51.
- ↑ Oxidation-Reduction Photochemistry of Metal Complexes in Solution Maverick, A. W.; Che, C.-M.; Nocera, D., G.; Winkler, J. R.; Gray, H. B., in Proceedings of the 4th International Conference on Photochemical Conversion and Storage of Solar Energy, J. Rabani, Editor. 1982: Jerusalem
- ↑ Electron-Transfer Kinetics of Pentammineruthenium(III)histidine-33-ferricytochrome c. Measurement of the Rate of Intramolecular Electron Transfer between Redox Centers Separated by 15 Å in a Protein Winkler, J. R.; Nocera, D. G.; Yocom, K. M.; Bordignon, E.; Gray, H. B. J. Am. Chem. Soc. 1982, 104, 5798-5800.
- ↑ Tryptophan-Accelerated Electron Flow through Proteins Shih, C.; Museth, A. K.; Abrahamsson, M.; Blanco-Rodriguez, A. M.; Di Bilio, A.; Sudhamsu, J.; Crane, B. R.; Ronayne, K. L.; Towrie, M.; Vlček, A.; Richards, J. H.; Winkler, J. R.; Gray, H. B. Science 2008, 320, 1760-1762.
- ↑ Probing Melittin Helix-coil Equilibria in Solutions and Vesicles Hartings, M. R.; Gray, H. B.; Winkler, J. R. J. Phys. Chem. B 2008, 112, 3202-3207.