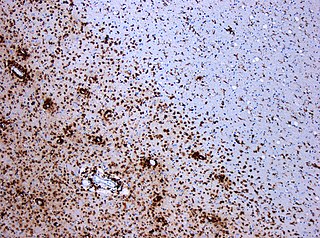
Multiplesclerosis (MS) is the most common demyelinating disease, in which the insulating covers of nerve cells in the brain and spinal cord are damaged. This damage disrupts the ability of parts of the nervous system to transmit signals, resulting in a range of signs and symptoms, including physical, mental, and sometimes psychiatric problems. Specific symptoms can include double vision, blindness in one eye, muscle weakness, and trouble with sensation or coordination. MS takes several forms, with new symptoms either occurring in isolated attacks or building up over time. In the relapsing forms of MS, between attacks, symptoms may disappear completely, although some permanent neurological problems often remain, especially as the disease advances.

Interferon beta-1a is a cytokine in the interferon family used to treat multiple sclerosis (MS). It is produced by mammalian cells, while interferon beta-1b is produced in modified E. coli. Some research indicates that interferon injections may result in an 18–38% reduction in the rate of MS relapses.

Fingolimod, sold under the brand name Gilenya, is an immunomodulating medication, mostly used for treating multiple sclerosis (MS). Fingolimod is a sphingosine-1-phosphate receptor modulator, which sequesters lymphocytes in lymph nodes, preventing them from contributing to an autoimmune reaction. It has been reported to reduce the rate of relapses in relapsing-remitting multiple sclerosis by approximately one-half over a two-year period.
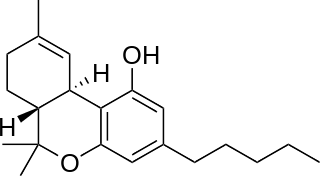
Nabiximols is a specific Cannabis extract that was approved in 2010 as a botanical drug in the United Kingdom. Nabiximols is sold as a mouth spray intended to alleviate neuropathic pain, spasticity, overactive bladder, and other symptoms of multiple sclerosis; it was developed by the UK company GW Pharmaceuticals. In 2019, it was proposed that following application of the spray, nabiximols is washed away from the oral mucosa by the saliva flow and ingested into the stomach, with subsequent absorption from the gastro-intestinal tract. Nabiximols is a combination drug standardized in composition, formulation, and dose. Its principal active components are the cannabinoids: tetrahydrocannabinol (THC) and cannabidiol (CBD). Each spray delivers a dose of 2.7 mg THC and 2.5 mg CBD.
Multiple sclerosis (MS) is a chronic inflammatory demyelinating disease that affects the central nervous system (CNS). Several therapies for it exist, although there is no known cure.

Laquinimod is an experimental immunomodulator developed by Active Biotech and Teva. It is being investigated as an oral treatment for multiple sclerosis (MS).
Research in multiple sclerosis may find new pathways to interact with the disease, improve function, curtail attacks, or limit the progression of the underlying disease. Many treatments already in clinical trials involve drugs that are used in other diseases or medications that have not been designed specifically for multiple sclerosis. There are also trials involving the combination of drugs that are already in use for multiple sclerosis. Finally, there are also many basic investigations that try to understand better the disease and in the future may help to find new treatments.

Dirucotide (also known as MBP8298) was developed by two research scientists (Dr. Kenneth G. Warren, MD, FRCP(C) & Ingrid Catz, Senior Scientist) at the University of Alberta for the treatment of Multiple Sclerosis (MS). Dirucotide is a synthetic peptide that consists of 17 amino acids linked in a sequence identical to that of a portion of human myelin basic protein (MBP). The sequence of these 17 amino acids is

Tumefactive multiple sclerosis is a condition in which the central nervous system of a person has multiple demyelinating lesions with atypical characteristics for those of standard multiple sclerosis (MS). It is called tumefactive as the lesions are "tumor-like" and they mimic tumors clinically, radiologically and sometimes pathologically.
Aaron E. Miller, M.D. is an American neurologist, the first Chairman of the Multiple Sclerosis section of the American Academy of Neurology (AAN) and recognized as a multiple sclerosis clinician.
Fred D. Lublin is an American neurologist and an authority on the treatment of multiple sclerosis. Along with colleagues at the National Multiple Sclerosis Society, his work redefined the clinical course definitions of MS.

Acorda Therapeutics, Inc. is an American biotechnology company based in Pearl River, New York. The company develops therapies that improve neurological function in people with Parkinson's disease, multiple sclerosis and other neurological disorders. Acorda Therapeutics manufactures and markets the drugs Inbrija and Ampyra (dalfampridine) in the United States.
The Patient Reported Outcome Indices for Multiple Sclerosis (PRIMUS) is a disease specific patient-reported outcome questionnaire which measures the quality of life (QoL) of patients with multiple sclerosis.

Ponesimod, sold under the brand name Ponvory, is a medication for the treatment of multiple sclerosis (MS).

Howard L. Weiner is an American neurologist, neuroscientist and immunologist who is also a writer and filmmaker. He performs clinical and basic research focused on multiple sclerosis (MS) and other neurologic diseases such as Alzheimer's Disease and Lou Gehrig's Disease (ALS). His work also focuses on autoimmune diseases such as diabetes. Weiner is the Robert L. Kroc Professor of Neurology at Harvard Medical School, director of the Brigham MS Center at the Brigham and Women's Hospital and co-director of the Ann Romney Center for Neurologic Diseases established in 2014, at the Brigham and Women's Hospital in Boston, Massachusetts.

Ozanimod, sold under the brand name Zeposia, is an immunomodulatory medication for the treatment of relapsing multiple sclerosis and ulcerative colitis. It acts as a sphingosine-1-phosphate (S1P) receptor agonist, sequestering lymphocytes to peripheral lymphoid organs and away from their sites of chronic inflammation.

Siponimod, sold under the brand name Mayzent, is a selective sphingosine-1-phosphate receptor modulator for oral use that is used for multiple sclerosis (MS). It is intended for once-daily oral administration.
There are several ways for pharmaceuticals for treating multiple sclerosis (MS) to reach the market.

Sphingosine-1-phosphate receptor modulators are a class of drugs used as immunomodulators, most notably in cases of multiple sclerosis.
Anne Cross is an American neurologist and neuroimmunologist and the Section Head of Neuroimmunology at Washington University School of Medicine in St. Louis, Missouri. Cross holds the Manny and Rosalyn Rosenthal–Dr. John L. Trotter Endowed Chair in Neuroimmunology at WUSTL School of Medicine and co-directs the John L Trotter Multiple Sclerosis Clinic at Barnes-Jewish Hospital. Cross is a leader in the field of neuroimmunology and was the first to discover the role of B cells in the pathogenesis of multiple sclerosis in animals and then in humans. Cross now develops novel imaging techniques to observe inflammation and demyelination in the central nervous systems of MS patients for diagnosis and disease management.











