Related Research Articles
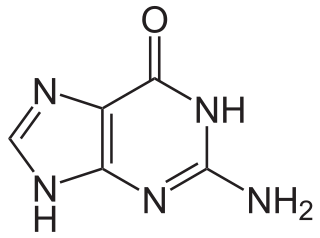
Guanine is one of the four main nucleobases found in the nucleic acids DNA and RNA, the others being adenine, cytosine, and thymine. In DNA, guanine is paired with cytosine. The guanine nucleoside is called guanosine.

The Miller–Urey experiment (or Miller experiment) is a famous chemistry experiment that simulated the conditions thought at the time (1952) to be present in the atmosphere of the early, prebiotic Earth, in order to test the hypothesis of the chemical origin of life under those conditions. The experiment used water (H2O), methane (CH4), ammonia (NH3), hydrogen (H2), and an electric arc (the latter simulating hypothesized lightning).

The RNA world is a hypothetical stage in the evolutionary history of life on Earth, in which self-replicating RNA molecules proliferated before the evolution of DNA and proteins. The term also refers to the hypothesis that posits the existence of this stage.

Alanine (symbol Ala or A), or α-alanine, is an α-amino acid that is used in the biosynthesis of proteins. It contains an amine group and a carboxylic acid group, both attached to the central carbon atom which also carries a methyl group side chain. Consequently, its IUPAC systematic name is 2-aminopropanoic acid, and it is classified as a nonpolar, aliphatic α-amino acid. Under biological conditions, it exists in its zwitterionic form with its amine group protonated (as −NH3+) and its carboxyl group deprotonated (as −CO2−). It is non-essential to humans as it can be synthesised metabolically and does not need to be present in the diet. It is encoded by all codons starting with GC (GCU, GCC, GCA, and GCG).

Nucleobases, also known as nitrogenous bases or often simply bases, are nitrogen-containing biological compounds that form nucleosides, which, in turn, are components of nucleotides, with all of these monomers constituting the basic building blocks of nucleic acids. The ability of nucleobases to form base pairs and to stack one upon another leads directly to long-chain helical structures such as ribonucleic acid (RNA) and deoxyribonucleic acid (DNA). Five nucleobases—adenine (A), cytosine (C), guanine (G), thymine (T), and uracil (U)—are called primary or canonical. They function as the fundamental units of the genetic code, with the bases A, G, C, and T being found in DNA while A, G, C, and U are found in RNA. Thymine and uracil are distinguished by merely the presence or absence of a methyl group on the fifth carbon (C5) of these heterocyclic six-membered rings. In addition, some viruses have aminoadenine (Z) instead of adenine. It differs in having an extra amine group, creating a more stable bond to thymine.

Stanley Lloyd Miller was an American chemist who made landmark experiments in the origin of life by demonstrating that a wide range of vital organic compounds can be synthesized by fairly simple chemical processes from inorganic substances. In 1952 he carried out the Miller–Urey experiment, which showed that complex organic molecules could be synthesised from inorganic precursors. The experiment was widely reported, and provided support for the idea that the chemical evolution of the early Earth had led to the natural synthesis of chemical building blocks of life from inanimate inorganic molecules. He has been described as the "father of prebiotic chemistry".
The iron–sulfur world hypothesis is a set of proposals for the origin of life and the early evolution of life advanced in a series of articles between 1988 and 1992 by Günter Wächtershäuser, a Munich patent lawyer with a degree in chemistry, who had been encouraged and supported by philosopher Karl R. Popper to publish his ideas. The hypothesis proposes that early life may have formed on the surface of iron sulfide minerals, hence the name. It was developed by retrodiction from extant biochemistry in conjunction with chemical experiments.

Leslie Eleazer Orgel FRS was a British chemist. He is known for his theories on the origin of life.

Joan Oró i Florensa was a Spanish biochemist, whose research has been of importance in understanding the origin of life. He participated in several NASA missions, including Apollo mission to the Moon and the Viking lander. He received the Oparin Medal, awarded by the International Astrobiology Society for his contributions to the field of origins of life.

Carbonaceous chondrites or C chondrites are a class of chondritic meteorites comprising at least 8 known groups and many ungrouped meteorites. They include some of the most primitive known meteorites. The C chondrites represent only a small proportion (4.6%) of meteorite falls.

The Murchison meteorite is a meteorite that fell in Australia in 1969 near Murchison, Victoria. It belongs to the carbonaceous chondrite class, a group of meteorites rich in organic compounds. Due to its mass and the fact that it was an observed fall, the Murchison meteorite is one of the most studied of all meteorites.
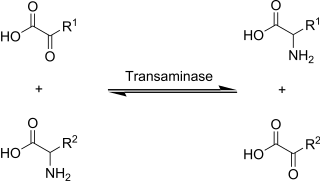
Transaminases or aminotransferases are enzymes that catalyze a transamination reaction between an amino acid and an α-keto acid. They are important in the synthesis of amino acids, which form proteins.

Nucleic acid analogues are compounds which are analogous to naturally occurring RNA and DNA, used in medicine and in molecular biology research. Nucleic acids are chains of nucleotides, which are composed of three parts: a phosphate backbone, a pentose sugar, either ribose or deoxyribose, and one of four nucleobases. An analogue may have any of these altered. Typically the analogue nucleobases confer, among other things, different base pairing and base stacking properties. Examples include universal bases, which can pair with all four canonical bases, and phosphate-sugar backbone analogues such as PNA, which affect the properties of the chain . Nucleic acid analogues are also called Xeno Nucleic Acid and represent one of the main pillars of xenobiology, the design of new-to-nature forms of life based on alternative biochemistries.
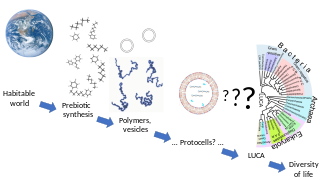
In biology, abiogenesis or the origin of life is the natural process by which life has arisen from non-living matter, such as simple organic compounds. The prevailing scientific hypothesis is that the transition from non-living to living entities on Earth was not a single event, but a process of increasing complexity involving the formation of a habitable planet, the prebiotic synthesis of organic molecules, molecular self-replication, self-assembly, autocatalysis, and the emergence of cell membranes. Many proposals have been made for different stages of the process.

In biochemistry, non-coded or non-proteinogenic amino acids are distinct from the 22 proteinogenic amino acids which are naturally encoded in the genome of organisms for the assembly of proteins. However, over 140 non-proteinogenic amino acids occur naturally in proteins and thousands more may occur in nature or be synthesized in the laboratory. Chemically synthesized amino acids can be called unnatural amino acids. Unnatural amino acids can be synthetically prepared from their native analogs via modifications such as amine alkylation, side chain substitution, structural bond extension cyclization, and isosteric replacements within the amino acid backbone. Many non-proteinogenic amino acids are important:

Sandra Pizzarello, D.Bi.Sc. was a Venetian biochemist known for her co-discovery of amino acid enantiomeric excess in carbonaceous chondrite meteorites. Her research interests concerned the characterization of meteoritic organic compounds in elucidating the evolution of planetary homochirality. Pizzarello was a project collaborator and co-investigator for the NASA Astrobiology Institute (NAI), the president of the International Society for the Study of the Origin of Life (2014-2017), and an emerita professor at Arizona State University (ASU).
Formamide-based prebiotic chemistry is a reconstruction of the beginnings of life on Earth, assuming that formamide could accumulate in sufficiently high amounts to serve as the building block and reaction medium for the synthesis of the first biogenic molecules.

The Urey instrument, or Urey: Mars Organic and Oxidant Detector was a developmental spacecraft instrument for detecting organic compounds including amino acids.
D-Amino acids are amino acids where the stereogenic carbon alpha to the amino group has the D-configuration. For most naturally-occurring amino acids, this carbon has the L-configuration. D-Amino acids are occasionally found in nature as residues in proteins. They are formed from ribosomally-derived D-amino acid residues.
In organic chemistry, secondary amino acids are amino acids which do not contain the amino group −NH2 but is rather a secondary amine. Secondary amino acids can be classified to cyclic acids, such as proline, and acyclic N-substituted amino acids.
References
- 1 2 3 Linda Ellis (2000). Archaeological Method and Theory: An Encyclopedia. Taylor & Francis. p. 67. ISBN 9780203801567.
- 1 2 "Curriculum Vitae: Jeffrey L. Bada" (PDF). Scripps Institution of Oceanography, UC San Diego. 2013. Retrieved September 9, 2013.
- 1 2 Claudia Dreifus (May 17, 2010). "A Marine Chemist Studies How Life Began". The New York Times. Retrieved September 10, 2013.
- ↑ Bada JL (1970). "Marine sediments: dating by the racemization of amino acids". Science. 170 (3959): 730–732. Bibcode:1970Sci...170..730B. doi:10.1126/science.170.3959.730. PMID 5479627. S2CID 6124313.
- ↑ Glavin DP, Bada JL, Brinton KL, McDonald GD (1999). "Amino acids in the Martian meteorite Nakhla". Proc Natl Acad Sci U S A. 96 (16): 8835–8839. Bibcode:1999PNAS...96.8835G. doi: 10.1073/pnas.96.16.8835 . PMC 17693 . PMID 10430856.
- ↑ Skelley AM, Scherer JR, Aubrey AD, Grover WH, Ivester RH, Ehrenfreund P, Grunthaner FJ, Bada JL, Mathies RA (2005). "Development and evaluation of a microdevice for amino acid biomarker detection and analysis on Mars". Proc Natl Acad Sci U S A. 102 (4): 1041–1046. Bibcode:2005PNAS..102.1041S. doi: 10.1073/pnas.0406798102 . PMC 545824 . PMID 15657130.
- ↑ Skelley AM, Cleaves HJ, Jayarajah CN, Bada JL, Mathies RA (2006). "Application of the Mars Organic Analyzer to nucleobase and amine biomarker detection". Astrobiology. 6 (6): 824–837. Bibcode:2006AsBio...6..824S. doi:10.1089/ast.2006.6.824. PMID 17155883.
- ↑ Douglas Fox (March 28, 2007). "Primordial Soup's On: Scientists Repeat Evolution's Most Famous Experiment". Scientific American. Retrieved September 10, 2013.
- ↑ Amina Khan (March 26, 2011). "New data mined from historic 'primordial soup' study". Los Angeles Times. Retrieved September 10, 2013.
- ↑ Johnson AP, Cleaves HJ, Dworkin JP, Glavin DP, Lazcano A, Bada JL (2008). "The Miller volcanic spark discharge experiment". Science. 322 (5900): 404. Bibcode:2008Sci...322..404J. doi:10.1126/science.1161527. PMID 18927386. S2CID 10134423.
- ↑ Miller SL (1953). "Production of amino acids under possible primitive earth conditions". Science. 117 (3046): 528–529. Bibcode:1953Sci...117..528M. doi:10.1126/science.117.3046.528. PMID 13056598.
- ↑ Bada JL (2013). "New insights into prebiotic chemistry from Stanley Miller's spark discharge experiments". Chem Soc Rev. 42 (5): 2186–2196. doi:10.1039/c3cs35433d. PMID 23340907.
- ↑ Parker ET, Cleaves HJ, Dworkin JP, Glavin DP, Callahan M, Aubrey A, Lazcano A, Bada JL (2011). "Primordial synthesis of amines and amino acids in a 1958 Miller H2S-rich spark discharge experiment". Proc Natl Acad Sci USA. 108 (12): 5526–5531. Bibcode:2011PNAS..108.5526P. doi: 10.1073/pnas.1019191108 . hdl:2060/20110013464. PMC 3078417 . PMID 21422282.
- ↑ Keim, Brandon (October 16, 2008). "Forgotten Experiment May Explain Origins of Life". Wired Magazine . Retrieved March 22, 2011.
- ↑ Steigerwald, Bill (October 16, 2008). "Volcanoes May Have Provided Sparks and Chemistry for First Life". NASA Goddard Space Flight Center . Retrieved March 22, 2011.