Protease inhibitors (PIs) are medications that act by interfering with enzymes that cleave proteins. Some of the most well known are antiviral drugs widely used to treat HIV/AIDS, hepatitis C and COVID-19. These protease inhibitors prevent viral replication by selectively binding to viral proteases and blocking proteolytic cleavage of protein precursors that are necessary for the production of infectious viral particles.

Vertex Pharmaceuticals Incorporated is an American biopharmaceutical company based in Boston, Massachusetts. It was one of the first biotech firms to use an explicit strategy of rational drug design rather than combinatorial chemistry. It maintains headquarters in Boston, Massachusetts, and three research facilities, in San Diego, California, and Milton Park, Oxfordshire, England.

Stuart Schreiber is an American chemist who is the Morris Loeb Research Professor at Harvard University, a co-founder of the Broad Institute, Howard Hughes Medical Institute Investigator, Emeritus, and a member of the National Academy of Sciences and National Academy of Medicine. He currently leads Arena BioWorks.
Venture philanthropy is a type of impact investment that takes concepts and techniques from venture capital finance and business management and applies them to achieving philanthropic goals. The term was first used in 1969 by John D. Rockefeller III to describe an imaginative and risk-taking approach to philanthropy that may be undertaken by charitable organizations.

Boceprevir is a protease inhibitor used to treat hepatitis caused by hepatitis C virus (HCV) genotype 1. It binds to the HCV nonstructural protein 3 active site.

Telaprevir (VX-950), marketed under the brand names Incivek and Incivo, is a pharmaceutical drug for the treatment of hepatitis C co-developed by Vertex Pharmaceuticals and Johnson & Johnson. It is a member of a class of antiviral drugs known as protease inhibitors. Specifically, telaprevir inhibits the hepatitis C viral enzyme NS3/4A serine protease. Telaprevir is only indicated for use against hepatitis C genotype 1 viral infections and has not been proven to be safe or effective when used for other genotypes of the virus. The standard therapy of pegylated interferon and ribavirin is less effective than telaprevir in those with genotype 1.
Many major physiological processes depend on regulation of proteolytic enzyme activity and there can be dramatic consequences when equilibrium between an enzyme and its substrates is disturbed. In this prospective, the discovery of small-molecule ligands, like protease inhibitors, that can modulate catalytic activities has an enormous therapeutic effect. Hence, inhibition of the HIV protease is one of the most important approaches for the therapeutic intervention in HIV infection and their development is regarded as major success of structure-based drug design. They are highly effective against HIV and have, since the 1990s, been a key component of anti-retroviral therapies for HIV/AIDS.

Ivacaftor is a medication used to treat cystic fibrosis in people with certain mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene, who account for 4–5% cases of cystic fibrosis. It is also included in combination medications, lumacaftor/ivacaftor, tezacaftor/ivacaftor, and elexacaftor/tezacaftor/ivacaftor which are used to treat people with cystic fibrosis.
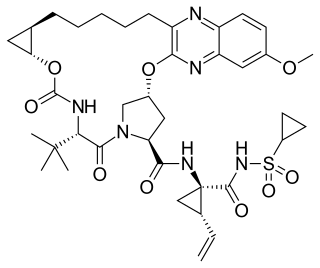
Grazoprevir is a drug approved for the treatment of hepatitis C. It was developed by Merck and completed Phase III trials, used in combination with the NS5A replication complex inhibitor elbasvir under the trade name Zepatier, either with or without ribavirin.

Lumacaftor/ivacaftor, sold under the brand name Orkambi among others, is a combination of lumacaftor and ivacaftor used to treat people with cystic fibrosis who have two copies of the F508del mutation. It is unclear if it is useful in cystic fibrosis due to other causes. It is taken by mouth.

Aurora Biosciences was a biotechnology company founded in 1995 in San Diego to commercialize fluorescence assays based on Roger Y. Tsien's discoveries concerning green fluorescent protein and its uses in basic research - work for which Tsien eventually won the 2008 Nobel Prize in chemistry along with two other chemists.
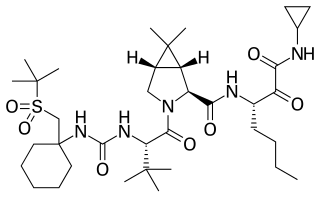
Narlaprevir, is an inhibitor of NS3/4A serine protease, intended for the treatment of chronic hepatitis C caused by genotype 1 virus in combination with other antiviral drugs.

Danoprevir (INN) is an orally available 15-membered macrocyclic peptidomimetic inhibitor of NS3/4A HCV protease. It contains acylsulfonamide, fluoroisoindole and tert-butyl carbamate moieties. Danoprevir is a clinical candidate based on its favorable potency profile against multiple HCV genotypes 1–6 and key mutants (GT1b, IC50 = 0.2–0.4 nM; replicon GT1b, EC50 = 1.6 nM). Danoprevir has been evaluated in an open-label, single arm clinical trial in combination with ritonavir for treating COVID-19 and favourably compared to lopinavir/ritonavir in a second trial.

Nancy A. Thornberry is the founding CEO and current chair, R&D at Kallyope Inc. in New York City. She previously worked with Merck Research Laboratories (MRL), joining the company in 1979 as a biochemist and retiring from the position of senior vice president and franchise head, diabetes and endocrinology in 2013.
John J. Talley is an American medicinal chemist who was the lead chemist in the discovery of the COX-2 selective nonsteroidal anti-inflammatory drug celecoxib and a co-inventor of amprenavir, a protease inhibitor used to treat HIV infection.

Jeffrey Leiden is an American physician, scientist and businessman who is the executive chairman of Vertex Pharmaceuticals, a biotechnology company based in Boston, Massachusetts. He was initially appointed to the board of directors of the company in 2009 and was CEO and president from February 2012 to March 2020.
Reshma Kewalramani, is the president and chief executive officer of Vertex Pharmaceuticals, a biotechnology company based in Boston, Massachusetts, as of April 1, 2020. She is the first female CEO of a large US biotech company. She was previously the chief medical officer and vice president of global medicines development and medical affairs at Vertex.
Peter Grootenhuis was a Dutch-American Medicinal Chemist. Grootenhuis was the Project Leader and Co-Inventor of Ivacaftor (VX-770), the first CFTR potentiator FDA approved drug to treat the underlying cause of Cystic Fibrosis (CF) in patients with certain mutations in the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) gene, who account for 4-5% of CF cases. Grootenhuis also led the Vertex team to subsequent discovery of Orkambi, the combination of Ivacaftor and Lumacaftor(VX-809), approved to treat CF in people with two copies of the F508del mutation. Most recently, Grootenhuis's team discovered Tezacaftor (VX-661) and Elexacaftor (VX-445), which in combination with Ivacaftor are the components of Trikafta, a drug approved by the FDA in 2019 to treat CF in more than 90% of CF patients. For Grootenhuis’ contributions to the discovery of these compounds, he was awarded the 2018 IUPAC Richter Prize, the American Chemical Society’s 2013 Heroes of Chemistry Award, and inducted into the American Chemical Society Division of Medicinal Chemistry Hall of Fame. Grootenhuis has contributed to the discovery of over 11 clinical candidates, co-authored more than 100 peer reviewed papers and is inventor of 65 + U.S Patents, and more than 50 EU Patents.
Paul Adrian Negulescu is an American–Romanian cell biologist. He is a Senior Vice President at American pharmaceutical company Vertex Pharmaceuticals. He received the 2022 Shaw Prize in Life science and medicine, together with Michael J. Welsh, for their work that uncovered the etiology of cystic fibrosis and developed effective medications.
Sabine Hadida is a pharmacologist and senior vice president at Vertex Pharmaceuticals. She works at Vertex's cystic fibrosis research center in San Diego. She was awarded the Breakthrough Prize in Life Sciences in 2024.












