Robert Howard Crabtree is a British-American chemist. He is serving as Conkey P. Whitehead Professor Emeritus of Chemistry at Yale University in the United States. He is a naturalized citizen of the United States. Crabtree is particularly known for his work on "Crabtree's catalyst" for hydrogenations, and his textbook on organometallic chemistry.
Organogermanium chemistry is the science of chemical species containing one or more C–Ge bonds. Germanium shares group 14 in the periodic table with carbon, silicon, tin and lead. Historically, organogermanes are considered as nucleophiles and the reactivity of them is between that of organosilicon and organotin compounds. Some organogermanes have enhanced reactivity compared with their organosilicon and organoboron analogues in some cross-coupling reactions.

Franz Hein was a German chemist and professor. He specialized in the chemistry of organic chromium and other metal compounds. He was the son of the artist Franz Johann Erich Hein (1863–1927).
Robert George Bergman is an American chemist. He is Professor of the Graduate School and Gerald E. K. Branch Distinguished Professor Emeritus at the University of California, Berkeley.
Melanie Sarah Sanford is an American chemist, currently the Moses Gomberg Distinguished University Professor of Chemistry and Arthur F. Thurnau Professor of Chemistry at the University of Michigan. She is a Fellow for the American Association for the Advancement of Science, and was elected a member of the National Academy of Sciences and the American Academy of Arts and Sciences in 2016. She has served as an executive editor of the Journal of the American Chemical Society since 2021, having been an associate editor of the since 2014.
A metal carbido complex is a coordination complex that contains a carbon atom as a ligand. They are analogous to metal nitrido complexes. Carbido complexes are a molecular subclass of carbides, which are prevalent in organometallic and inorganic chemistry. Carbido complexes represent models for intermediates in Fischer–Tropsch synthesis, olefin metathesis, and related catalytic industrial processes. Ruthenium-based carbido complexes are by far the most synthesized and characterized to date. Although, complexes containing chromium, gold, iron, nickel, molybdenum, osmium, rhenium, and tungsten cores are also known. Mixed-metal carbides are also known.
Jaqueline Kiplinger is an American inorganic chemist who specializes in organometallic actinide chemistry. Over the course of her career, she has done extensive work with fluorocarbons and actinides. She is currently a Fellow of the Materials Synthesis and Integrated Devices group in the Materials Physics and Applications Division of Los Alamos National Laboratory (LANL). Her current research interests are focused on the development of chemistry for the United States’ national defense and energy needs.
Russell P. Hughes an American/British chemist, is the Frank R. Mori Professor Emeritus and Research Professor in the Department of Chemistry at Dartmouth College. His research interests are in organometallic chemistry, with emphasis on the chemistry of transition metal complexes interacting with fluorocarbons. His research group’s work in this area led to several creative syntheses of complexes of transition metal and perfluorinated hydrocarbon fragments.
Parisa Mehrkhodavandi is a Canadian chemist and Professor of Chemistry at the University of British Columbia (UBC). Her research focuses on the design of new catalysts that can effect polymerization of sustainably sourced or biodegradable polymers.
Clark Landis is an American chemist, whose research focuses on organic and inorganic chemistry. He is currently a Professor of Chemistry at the University of Wisconsin–Madison. He was awarded the ACS Award in Organometallic Chemistry in 2010, and is a fellow of the American Chemical Society and the American Association for the Advancement of Science.
Christopher "Kit" Colin Cummins is an American chemist, currently the Henry Dreyfus Professor at the Massachusetts Institute of Technology. He has made contributions to the coordination chemistry of transition metal nitrides, phosphides, and carbides.
William B. Tolman an American inorganic chemist focusing on the synthesis and characterization of model bioinorganic systems, and organometallic approaches towards polymer chemistry. He has served as Editor in Chief of the ACS journal Inorganic Chemistry, and as a Senior Investigator at the NSF Center for Sustainable Polymers. Tolman is a Fellow of the American Association for the Advancement of Science and the American Chemical Society.
T. Don Tilley is a professor of chemistry at the University of California, Berkeley.
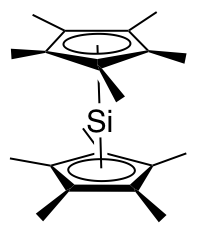
Decamethylsilicocene, (C5Me5)2Si, is a group 14 sandwich compound. It is an example of a main-group cyclopentadienyl complex; these molecules are related to metallocenes but contain p-block elements as the central atom. It is a colorless, air sensitive solid that sublimes under vacuum.
Jonas C. Peters is the Bren Professor of Chemistry at the California Institute of Technology and Director of the Resnick Sustainability Institute. He has contributed to the development of catalysts and photocatalysts relevant to small molecule activation.
Organotechnetium chemistry is the science of describing the physical properties, synthesis, and reactions of organotechnetium compounds, which are organometallic compounds containing carbon-to-technetium chemical bonds. The most common organotechnetium compounds are coordination complexes used as radiopharmaceutical imaging agents.
Karsten Meyer is a German inorganic chemist and Chair of Inorganic and General Chemistry at the Friedrich-Alexander University of Erlangen-Nürnberg (FAU). His research involves the coordination chemistry of transition metals as well as uranium coordination chemistry, small molecule activation with these coordination complexes, and the synthesis of new chelating ligands. He is the 2017 recipient of the Elhuyar-Goldschmidt Award of the Spanish Royal Society of Chemistry, the Ludwig-Mond Award of the Royal Society of Chemistry, and the L.A. Chugaev Commemorative Medal of the Russian Academy of Sciences, among other awards. He also serves as an Associate Editor of the journal Organometallics since 2014.

Paula L. Diaconescu is a Romanian-American chemistry professor at the University of California, Los Angeles. She is known for her research on the synthesis of redox active transition metal complexes, the synthesis of lanthanide complexes, metal-induced small molecule activation, and polymerization reactions. She is a fellow of the American Association for the Advancement of Science.
Suzanne Cathleen Bart an American chemist who is a professor of inorganic chemistry at Purdue University. Her group's research focuses on actinide organometallic chemistry, and especially the characterization of low-valent organouranium complexes, actinide complexes with redox-active ligands, and discovery of new reactions that utilize these compounds. Bart's research has applications in the development of carbon-neutral fuel sources and the remediation of polluted sites.

Disulfidobis(tricarbonyliron), or Fe2(μ-S2)(CO)6, is an organometallic molecule used as a precursor in the synthesis of iron-sulfur compounds. Popularized as a synthetic building block by Dietmar Seyferth, Fe2(μ-S2)(CO)6 is commonly used to make mimics of the H-cluster in [FeFe]-hydrogenase. Much of the reactivity of Fe2(μ-S2)(CO)6 proceeds through its sulfur-centered dianion, [Fe2(μ-S)2(CO)2]2-.