
Kaposi's sarcoma-associated herpesvirus (KSHV) is the ninth known human herpesvirus; its formal name according to the International Committee on Taxonomy of Viruses (ICTV) is Human gammaherpesvirus 8, or HHV-8 in short. Like other herpesviruses, its informal names are used interchangeably with its formal ICTV name. This virus causes Kaposi's sarcoma, a cancer commonly occurring in AIDS patients, as well as primary effusion lymphoma, HHV-8-associated multicentric Castleman's disease and KSHV inflammatory cytokine syndrome. It is one of seven currently known human cancer viruses, or oncoviruses. Even after so many years of discovery of KSHV/HHV8, there is no known cure for KSHV associated tumorigenesis.
An oncovirus is a virus that can cause cancer. This term originated from studies of acutely transforming retroviruses in the 1950–60s, when the term "oncornaviruses" was used to denote their RNA virus origin. With the letters "RNA" removed, it now refers to any virus with a DNA or RNA genome causing cancer and is synonymous with "tumor virus" or "cancer virus". The vast majority of human and animal viruses do not cause cancer, probably because of longstanding co-evolution between the virus and its host. Oncoviruses have been important not only in epidemiology, but also in investigations of cell cycle control mechanisms such as the retinoblastoma protein.
An internal ribosome entry site, abbreviated IRES, is an RNA element that allows for translation initiation in a cap-independent manner, as part of the greater process of protein synthesis. In eukaryotic translation, initiation typically occurs at the 5' end of mRNA molecules, since 5' cap recognition is required for the assembly of the initiation complex. The location for IRES elements is often in the 5'UTR, but can also occur elsewhere in mRNAs.
Patrick S. Moore is an Irish and American virologist and epidemiologist who co-discovered together with his wife, Yuan Chang, two different human viruses causing the AIDS-related cancer Kaposi's sarcoma and the skin cancer Merkel cell carcinoma. The couple met while in medical school together and were married in 1989 while they pursued fellowships at different universities.

Interferon regulatory factors (IRF) are proteins which regulate transcription of interferons. They are used in the JAK-STAT signaling pathway. Interferon regulatory factors contain a conserved N-terminal region of about 120 amino acids, which folds into a structure that binds specifically to the interferon consensus sequence (ICS), which is located upstream of the interferon genes. The remaining parts of the interferon regulatory factor sequence vary depending on the precise function of the protein. The Kaposi sarcoma herpesvirus, KSHV, is a cancer virus that encodes four different IRF-like genes; including vIRF1, which is a transforming oncoprotein that inhibits type 1 interferon activity. In addition, the expression of IRF genes is under epigenetic regulation by promoter DNA methylation.

Viral entry is the earliest stage of infection in the viral life cycle, as the virus comes into contact with the host cell and introduces viral material into the cell. The major steps involved in viral entry are shown below. Despite the variation among viruses, there are several shared generalities concerning viral entry.
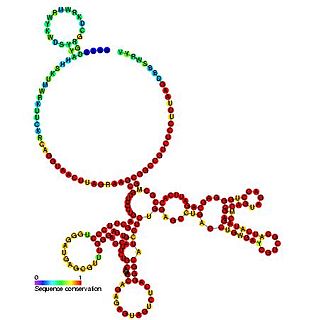
The Epstein–Barr virus nuclear-antigen internal ribosome entry site is an internal ribosome entry site (IRES) that is found in an exon in the 5' untranslated region of the Epstein–Barr virus nuclear antigen 1 (EBNA1) gene. The EBNA IRES allows EBNA1 translation to occur under situations where initiation from the 5' cap structure and ribosome scanning is reduced. It is thought that the EBNA IRES is necessary for the regulation of latent-gene expression.

This family represents the internal ribosome entry site (IRES) of the hepatitis A virus. HAV IRES is a 450 nucleotide long sequence located in the 735 nt long 5’ UTR of Hepatitis A viral RNA genome. IRES elements allow cap and end-independent translation of mRNA in the host cell. The IRES achieves this by mediating the internal initiation of translation by recruiting a ribosomal 40S pre-initiation complex directly to the initiation codon and eliminates the requirement for eukaryotic initiation factor, eIF4F.

Cyclin-O is a protein that in humans is encoded by the CCNO gene.

Membrane-associated tyrosine- and threonine-specific cdc2-inhibitory kinase also known as Myt1 kinase is an enzyme that in humans is encoded by the PKMYT1 gene.
Murid gammaherpesvirus 68 (MuHV-68) is an isolate of the virus species Murid gammaherpesvirus 4, a member of the genus Rhadinovirus. It is a member of the subfamily Gammaherpesvirinae in the family of Herpesviridae. MuHV-68 serves as a model for study of human gammaherpesviruses which cause significant human disease including B-cell lymphoma and Kaposi's sarcoma. The WUMS strain of MuHV-68 was fully sequenced and annotated in 1997, and the necessity of most of its genes in viral replication was characterized by random transposon mutagenesis.
The latency-associated nuclear antigen (LANA-1) or latent nuclear antigen , is a Kaposi's sarcoma-associated herpesvirus (KSHV) latent protein initially found by Moore and colleagues as a speckled nuclear antigen present in primary effusion lymphoma cells that reacts with antibodies from patients with KS. It is the most immunodominant KSHV protein identified by Western-blotting as 222–234 kDa double bands migrate slower than the predicted molecular weight. LANA has been suspected of playing a crucial role in modulating viral and cellular gene expression. It is commonly used as an antigen in blood tests to detect antibodies in persons that have been exposed to KSHV.

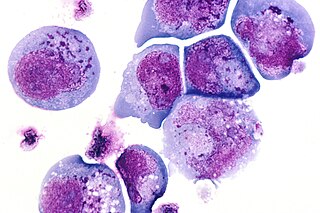
Kaposi's sarcoma (KS) is a type of cancer that can form masses in the skin, lymph nodes, or other organs. The skin lesions are usually purple in color. They can occur singularly, in a limited area, or be widespread. It may worsen either gradually or quickly. Lesions may be flat or raised. Human herpesvirus 8 (HHV8) is found in the lesions of all those who are affected. Risk factors include poor immune function, either as a result of disease or specific medications, and chronic lymphedema.

Red clover necrotic mosaic virus (RCNMV) contains several structural elements present within the 3′ and 5′ untranslated regions (UTR) of the genome that enhance translation. In eukaryotes transcription is a prerequisite for translation. During transcription the pre-mRNA transcript is processes where a 5′ cap is attached onto mRNA and this 5′ cap allows for ribosome assembly onto the mRNA as it acts as a binding site for the eukaryotic initiation factor eIF4F. Once eIF4F is bound to the mRNA this protein complex interacts with the poly(A) binding protein which is present within the 3′ UTR and results in mRNA circularization. This multiprotein-mRNA complex then recruits the ribosome subunits and scans the mRNA until it reaches the start codon. Transcription of viral genomes differs from eukaryotes as viral genomes produce mRNA transcripts that lack a 5’ cap site. Despite lacking a cap site viral genes contain a structural element within the 5’ UTR known as an internal ribosome entry site (IRES). IRES is a structural element that recruits the 40s ribosome subunit to the mRNA within close proximity of the start codon.
Eva Henriette Gottwein is a virologist and Assistant Professor of Microbiology-Immunology at Northwestern University Feinberg School of Medicine in Chicago, Illinois. The main focus of her research is the role of viral miRNAs involved in herpesviral oncogenesis. Gottwein is member of Lurie Cancer Center at Robert H. Lurie Comprehensive Cancer Center of Northwestern University. Her contributions as a member include the focus on how encoded miRNAs target and function in the human oncogenic herpesvirus Kaposi's sarcoma-associated herpesvirus known as KSHV.
Human Herpes Viruses, also known as HHVs, are part of a family of DNA viruses that cause several diseases in humans. One of the most notable functions of this virus family is their ability to enter a latent phase and lay dormant within animals for extended periods of time. The mechanism that controls this is very complex because expression of viral proteins during latency is decreased a great deal, meaning that the virus must have transcription of its genes repressed. There are many factors and mechanisms that control this process and epigenetics is one way this is accomplished. Epigenetics refers to persistent changes in expression patterns that are not caused by changes to the DNA sequence. This happens through mechanisms such as methylation and acetylation of histones, DNA methylation, and non-coding RNAs (ncRNA). Altering the acetylation of histones creates changes in expression by changing the binding affinity of histones to DNA, making it harder or easier for transcription machinery to access the DNA. Methyl and acetyl groups can also act as binding sites for transcription factors and enzymes that further modify histones or alter the DNA itself.

Dean Hamilton Kedes is an American scientist in the field of virology and current director of the medical scientist training program at the University of Virginia school of medicine.

Rhopalosiphum padi virus (RhPV) is a member of Dicistroviridae family, which includes cricket paralysis virus (CrPV), Plautia stali intestine virus and Drosophila C virus. Its 5'UTR region contains an internal ribosome entry site (IRES) element with a cross-kingdom activity. It can function efficiently in mammalian, plant and insect translation systems. Testing of R. padi aphids collected from different sites in Sweden revealed the presence of RhPV in wild aphid populations for the first time in Europe. Virus could be detected in several life stages of R. padi, including sexual individuals and eggs, establishing an over-wintering route for the virus.
Human herpesvirus 8 associated multicentric Castleman disease is a subtype of Castleman disease, a group of rare lymphoproliferative disorders characterized by lymph node enlargement, characteristic features on microscopic analysis of enlarged lymph node tissue, and a range of symptoms and clinical findings.
HSV epigenetics is the epigenetic modification of herpes simplex virus (HSV) genetic code.












