Keanumycins are a group of chemical compounds isolated from bacteria in the genus Pseudomonas . [1] They are classified as nonribosomal lipopeptides and they have a variety of antimicrobial activities. Keanumycin A is active against the amoebas Dictyostelium discoideum (IC50 = 4.4 nM), Acanthamoeba castellanii (IC50 = 2.0 μM), and Acanthamoeba comandoni (IC50 = 3.1 μM) which cause infections in humans. [1]
Fermentation broth containing keanumycins is effective against the fungus Botrytis cinerea and can be directly applied to plants to stop the development of Botrytis blight. [1]
Researchers at the Hans Knöll Institute who first isolated and characterized these compounds named them after the actor Keanu Reeves because their deadliness was perceived to be comparable to Reeves in his film roles. [2] [3]

Peptides are short chains of amino acids linked by peptide bonds. A polypeptide is a longer, continuous, unbranched peptide chain. Polypeptides that have a molecular mass of 10,000 Da or more are called proteins. Chains of fewer than twenty amino acids are called oligopeptides, and include dipeptides, tripeptides, and tetrapeptides.

Secondary metabolites, also called specialised metabolites, toxins, secondary products, or natural products, are organic compounds produced by any lifeform, e.g. bacteria, fungi, animals, or plants, which are not directly involved in the normal growth, development, or reproduction of the organism. Instead, they generally mediate ecological interactions, which may produce a selective advantage for the organism by increasing its survivability or fecundity. Specific secondary metabolites are often restricted to a narrow set of species within a phylogenetic group. Secondary metabolites often play an important role in plant defense against herbivory and other interspecies defenses. Humans use secondary metabolites as medicines, flavourings, pigments, and recreational drugs.

Botrytis cinerea is a necrotrophic fungus that affects many plant species, although its most notable hosts may be wine grapes. In viticulture, it is commonly known as "botrytis bunch rot"; in horticulture, it is usually called "grey mould" or "gray mold".

Phytoalexins are antimicrobial substances, some of which are antioxidative as well. They are defined, not by their having any particular chemical structure or character, but by the fact that they are defensively synthesized de novo by plants that produce the compounds rapidly at sites of pathogen infection. In general phytoalexins are broad spectrum inhibitors; they are chemically diverse, and different chemical classes of compounds are characteristic of particular plant taxa. Phytoalexins tend to fall into several chemical classes, including terpenoids, glycosteroids, and alkaloids; however the term applies to any phytochemicals that are induced by microbial infection.

Surfactin is a cyclic lipopeptide, commonly used as an antibiotic for its capacity as a surfactant. It is an amphiphile capable of withstanding hydrophilic and hydrophobic environments. The Gram-positive bacterial species Bacillus subtilis produces surfactin for its antibiotic effects against competitors. Surfactin showcases antibacterial, antiviral, antifungal, and hemolytic effects.

Aleph is a psychedelic hallucinogenic drug and a substituted amphetamine of the phenethylamine class of compounds, which can be used as an entheogen. It was first synthesized by Alexander Shulgin, who named it after the first letter of the Hebrew alphabet. In his book PiHKAL, Shulgin lists the dosage range as 5–10 mg, with effects typically lasting for 6 to 8 hours.
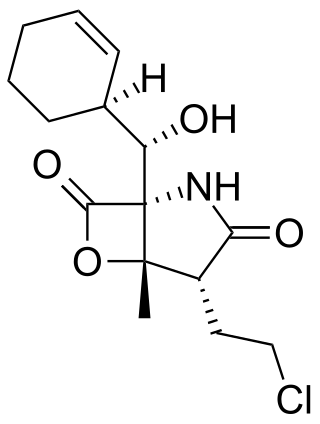
Salinosporamide A (Marizomib) is a potent proteasome inhibitor being studied as a potential anticancer agent. It entered phase I human clinical trials for the treatment of multiple myeloma, only three years after its discovery in 2003. This marine natural product is produced by the obligate marine bacteria Salinispora tropica and Salinispora arenicola, which are found in ocean sediment. Salinosporamide A belongs to a family of compounds, known collectively as salinosporamides, which possess a densely functionalized γ-lactam-β-lactone bicyclic core.

Fluorenol, also known as hydrafinil, is an alcohol derivative of fluorene. In the most significant isomer, fluoren-9-ol or 9-hydroxyfluorene, the hydroxy group is located on the bridging carbon between the two benzene rings. Hydroxyfluorene can be converted to fluorenone by oxidation. It is a white-cream colored solid at room temperature.

Morin is a yellow chemical compound that can be isolated from Maclura pomifera (Osage orange), Maclura tinctoria (old fustic), and from leaves of Psidium guajava (common guava). In a preclinical in vitro study, morin was found to be a weak inhibitor of fatty acid synthase with an IC50 of 2.33 μM. Morin was also found to inhibit amyloid formation by islet amyloid polypeptide (or amylin) and disaggregate amyloid fibers.

Codinaeopsin is an antimalarial isolated from a fungal isolate found in white yemeri trees (Vochysia guatemalensis) in Costa Rica. It is reported to have bioactivity against Plasmodium falciparum with an IC50 = 2.3 μg/mL (4.7 μM). Pure codinaeopsin was reported to be isolated with a total yield of 18 mg/mL from cultured fungus. The biosynthesis of codinaeopsin involves a polyketide synthase-nonribosomal peptide synthetase (PKS-NRPS) hybrid.
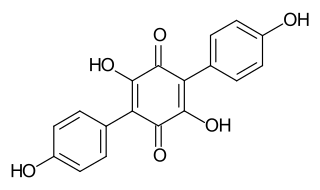
Atromentin is a natural chemical compound found in Agaricomycetes fungi in the orders Agaricales and Thelephorales. It can also be prepared by laboratory synthesis. Chemically, it is a polyphenol and a benzoquinone.
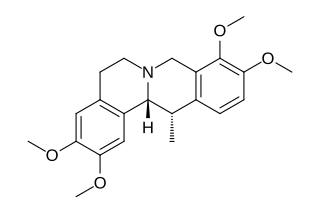
Corydaline is an acetylcholinesterase inhibitor isolated from Corydalis yanhusuo.
Teixobactin is a peptide-like secondary metabolite of some species of bacteria, that kills some gram-positive bacteria. It appears to belong to a new class of antibiotics, and harms bacteria by binding to lipid II and lipid III, important precursor molecules for forming the cell wall.
Guineesine is a compound isolated from long pepper and black pepper.

Apparicine is a monoterpenoid tricyclic indole alkaloid. It is named after Apparicio Duarte, a Brazilian botanist who studied the Aspidosperma species from which apparicine was first isolated. It was the first member of the vallesamine group of indole alkaloids to be isolated and have its structure established, which was first published in 1965. It has also been known by the synonyms gomezine, pericalline, and tabernoschizine.

Pentabromopseudilin, the first reported marine microbial antibiotic, is a bioactive natural product that contains a highly halogenated 2-arrylpyrrole moiety. Pentabromopseudilin (PBP) is a unique hybrid bromophenol-bromopyrrole compound that is made up of over 70% bromine atoms, contributing to its potent bioactivity. PBP was first isolated from Pseudomonas bromoutilis, and has since been found to be produced by other marine microbes, including Alteromonas luteoviolaceus, Chromobacteria, and Pseudoalteromonas spp.
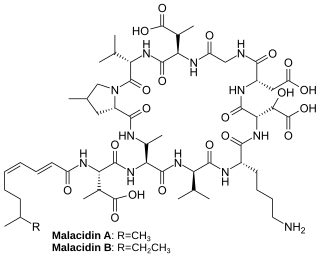
Malacidins are a class of chemicals made by bacteria found in soil that can kill Gram-positive bacteria. Their activity appears to be dependent on calcium. The discovery of malacidins was published in 2018.
A proteolipid is a protein covalently linked to lipid molecules, which can be fatty acids, isoprenoids or sterols. The process of such a linkage is known as protein lipidation, and falls into the wider category of acylation and post-translational modification. Proteolipids are abundant in brain tissue, and are also present in many other animal and plant tissues. They include ghrelin, a peptide hormone associated with feeding. Many proteolipids are composed of proteins covalenently bound to fatty acid chains, often granting them an interface for interacting with biological membranes. They are not to be confused with lipoproteins, a kind of spherical assembly made up of many molecules of lipids and some apolipoproteins.

Gallinamide A is potent and selective inhibitor of the human cysteine protease Cathepsin L1 that was first used as a moderate antimalarial agent. Gallinamide A is produced by marine cyanobacteria from Schizothrix species and Symploca sp. which have also shown to have possible anticancer agent, infectious diseases like leishmaniasis, trypanosomiasis and possible uses in Alzheimer's disease, among others.
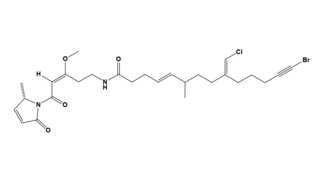
Jamaicamide A is a lipopeptide isolated from the cyanobacterium Moorea producens, formerly known as Lyngbya majuscula. Jamaicamide A belongs to a family of compounds collectively called jamaicamides, which are sodium channel blockers with potent neurotoxicity in a cellular model. Jamaicamide A has several unusual functionalities, including an alkynyl bromide, vinyl chloride, β-methoxy eneone system, and pyrrolinone ring.