Related Research Articles

Antimony is a chemical element; it has symbol Sb (from Latin stibium) and atomic number 51. A lustrous grey metal or metalloid, it is found in nature mainly as the sulfide mineral stibnite (Sb2S3). Antimony compounds have been known since ancient times and were powdered for use as medicine and cosmetics, often known by the Arabic name kohl. The earliest known description of the metalloid in the West was written in 1540 by Vannoccio Biringuccio.
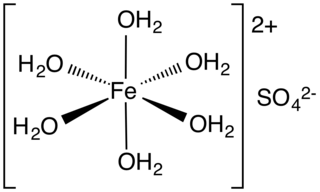
Iron(II) sulfate (British English: iron(II) sulphate) or ferrous sulfate denotes a range of salts with the formula FeSO4·xH2O. These compounds exist most commonly as the heptahydrate (x = 7) but several values for x are known. The hydrated form is used medically to treat or prevent iron deficiency, and also for industrial applications. Known since ancient times as copperas and as green vitriol (vitriol is an archaic name for hydrated sulfate minerals), the blue-green heptahydrate (hydrate with 7 molecules of water) is the most common form of this material. All the iron(II) sulfates dissolve in water to give the same aquo complex [Fe(H2O)6]2+, which has octahedral molecular geometry and is paramagnetic. The name copperas dates from times when the copper(II) sulfate was known as blue copperas, and perhaps in analogy, iron(II) and zinc sulfate were known respectively as green and white copperas.

A pnictogen is any of the chemical elements in group 15 of the periodic table. Group 15 is also known as the nitrogen group or nitrogen family. Group 15 consists of the elements nitrogen (N), phosphorus (P), arsenic (As), antimony (Sb), bismuth (Bi), and moscovium (Mc).
Crimson is a rich, deep red color, inclining to purple. It originally meant the color of the kermes dye produced from a scale insect, Kermes vermilio, but the name is now sometimes also used as a generic term for slightly bluish-red colors that are between red and rose. It is the national color of Nepal.
Kermes may refer to :

Stibnite, sometimes called antimonite, is a sulfide mineral with the formula Sb2S3. This soft grey material crystallizes in an orthorhombic space group. It is the most important source for the metalloid antimony. The name is derived from the Greek στίβι stibi through the Latin stibium as the former name for the mineral and the element antimony.

Vermilion is a color family and pigment most often used between antiquity and the 19th century from the powdered mineral cinnabar. It is synonymous with red orange, which often takes a modern form, but is 11% brighter.

Realgar, also known as ″arsenic blende″, ″ruby sulphur″ or ″ruby of arsenic″, is an arsenic sulfide mineral with the chemical formula α-As4S4. It is a soft, sectile mineral occurring in monoclinic crystals, or in granular, compact, or powdery form, often in association with the related mineral, orpiment. It is orange-red in color, melts at 320 °C, and burns with a bluish flame releasing fumes of arsenic and sulfur. Realgar is soft with a Mohs hardness of 1.5 to 2 and has a specific gravity of 3.5. Its streak is orange colored. It is trimorphous with pararealgar and bonazziite.

Valentinite is an antimony oxide mineral with formula Sb2O3. Valentinite crystallizes in the orthorhombic system and typically forms as radiating clusters of euhedral crystals or as fibrous masses. It is colorless to white with occasional shades or tints of yellow and red. It has a Mohs hardness of 2.5 to 3 and a specific gravity of 5.76. Valentinite occurs as a weathering product of stibnite and other antimony minerals. It is dimorphous with the isometric antimony oxide senarmontite.

Scarlet is a bright red color, sometimes with a slightly orange tinge. In the spectrum of visible light, and on the traditional color wheel, it is one-quarter of the way between red and orange, slightly less orange than vermilion.

Alchermes is a type of Italian liqueur prepared by infusing neutral spirits with sugar, cinnamon, cloves, nutmeg, and vanilla, and other herbs and flavoring agents. Its most striking characteristic is its scarlet color, obtained by the addition of Kermes, a small scale insect from which the drink derives its name. Several proprietary variants are commercially available, where the coloring agent is a coal tar-derived dye such as E124 or E126, with alcoholic contents ranging from 21 to 32%. Its chief use is in coloring pastry, although a quick dessert is sometimes made by adding it to custard cream and sugar. In the Italian pudding zuppa inglese, sponge cake or ladyfingers soaked in this liqueur are a major ingredient.

Kermes is a red dye derived from the dried bodies of the females of a scale insect in the genus Kermes, primarily Kermes vermilio. The Kermes insects are native in the Mediterranean region and are parasites living on the sap of the host plant, the Kermes oak and the Palestine oak.

Kermesite or antimony oxysulfide is also known as red antimony or purpur blende (Sb2S2O). The mineral's color ranges from cherry red to a dark red to a black. Kermesite is the result of partial oxidation between stibnite (Sb2S3) and other antimony oxides such as valentinite (Sb2O3) or stibiconite (Sb3O6(OH)). Under certain conditions with oxygenated fluids the transformation of all sulfur to oxygen would occur but kermesite occurs when that transformation is halted.

Natural dyes are dyes or colorants derived from plants, invertebrates, or minerals. The majority of natural dyes are vegetable dyes from plant sources—roots, berries, bark, leaves, and wood—and other biological sources such as fungi.

Dyeing is the craft of imparting colors to textiles in loose fiber, yarn, cloth or garment form by treatment with a dye. Archaeologists have found evidence of textile dyeing with natural dyes dating back to the Neolithic period. In China, dyeing with plants, barks and insects has been traced back more than 5,000 years. Natural insect dyes such as Tyrian purple and kermes and plant-based dyes such as woad, indigo and madder were important elements of the economies of Asia and Europe until the discovery of man-made synthetic dyes in the mid-19th century. Synthetic dyes quickly superseded natural dyes for the large-scale commercial textile production enabled by the Industrial Revolution, but natural dyes remained in use by traditional cultures around the world.

Native antimony is a mineral belonging to the group of native elements, with properties equivalent to those of the antimony element obtained by processing its ores. The name comes from the Latin antimonium. For centuries, the term antimony was also used to refer to stibnite or antimonite, the most common mineral containing this element, from which it was typically extracted. In mineralogy, the official name is simply antimony, although, as with other native elements, it is often referred to as native antimony to avoid ambiguity. It is unclear where native antimony was first discovered, although the Sala silver mine in Västmanland, Sweden, is considered the type locality.
The color red is the longest wavelength of light discernable to the human eye, with a range of between 620 and 750 nanometers. Red was commonly the first color term added to languages after the colors of black and white. As well as this, the color was the first color to be used by humans. Because of this, certain languages used the word for the color red to simply be the word for any color visible to the human eye.

Red pigments are materials, usually made from minerals, used to create the red colors in painting and other arts. The color of red and other pigments is determined by the way it absorbs certain parts of the spectrum of visible light and reflects the others. The brilliant opaque red of vermillion, for example, results because vermillion reflects the major part of red light, but absorbs the blue, green and yellow parts of white light.

Arsenic blende or Arsenblende is a trivial name that has partially fallen out of scientific use, used by mineralogists, as well as representatives of mining and craft professions in relation to at least two similar ore minerals — orpiment and realgar, in composition — arsenic sulfides.
References
- ↑ Chisholm, Hugh, ed. (1911). . Encyclopædia Britannica . Vol. 15 (11th ed.). Cambridge University Press. p. 746.