Related Research Articles

Kyanite is a typically blue aluminosilicate mineral, found in aluminium-rich metamorphic pegmatites and sedimentary rock. It is the high pressure polymorph of andalusite and sillimanite, and the presence of kyanite in metamorphic rocks generally indicates metamorphism deep in the Earth's crust. Kyanite is also known as disthene or cyanite.

Chondrodite is a nesosilicate mineral with formula (Mg,Fe)
5(SiO
4)
2(F,OH,O)
2. Although it is a fairly rare mineral, it is the most frequently encountered member of the humite group of minerals. It is formed in hydrothermal deposits from locally metamorphosed dolomite. It is also found associated with skarn and serpentinite. It was discovered in 1817 at Pargas in Finland, and named from the Greek for "granule", which is a common habit for this mineral.

Zanazziite is a complex hydrated phosphate mineral from the roscherite group. It is a magnesium beryllium phosphate mineral. Zanazziite arises as barrel-shaped crystals and can reach up to 4 mm. It grows alongside quartz minerals. It is found in the crevices of Lavra da Ilha pegmatite, near Taquaral, in northeastern Minas Gerais, Brazil. Zanazziite is named after Pier F. Zanazzi. Zanazziite has an ideal chemical formula of Ca2Mg5Be4(PO4)6(OH)4·6H2O.
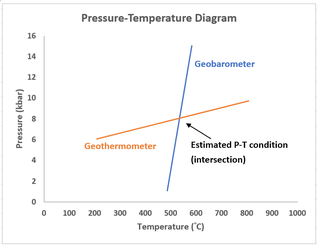
Geothermobarometry is the methodology for estimating the pressure and temperature history of rocks. Geothermobarometry is a combination of geobarometry, where the pressure attained by a mineral assemblage is estimated, and geothermometry where the temperature attained by a mineral assemblage is estimated.

Ferrihydrite (Fh) is a widespread hydrous ferric oxyhydroxide mineral at the Earth's surface, and a likely constituent in extraterrestrial materials. It forms in several types of environments, from freshwater to marine systems, aquifers to hydrothermal hot springs and scales, soils, and areas affected by mining. It can be precipitated directly from oxygenated iron-rich aqueous solutions, or by bacteria either as a result of a metabolic activity or passive sorption of dissolved iron followed by nucleation reactions. Ferrihydrite also occurs in the core of the ferritin protein from many living organisms, for the purpose of intra-cellular iron storage.

Wadsleyite is an orthorhombic mineral with the formula β-(Mg,Fe)2SiO4. It was first found in nature in the Peace River meteorite from Alberta, Canada. It is formed by a phase transformation from olivine (α-(Mg,Fe)2SiO4) under increasing pressure and eventually transforms into spinel-structured ringwoodite (γ-(Mg,Fe)2SiO4) as pressure increases further. The structure can take up a limited amount of other bivalent cations instead of magnesium, but contrary to the α and γ structures, a β structure with the sum formula Fe2SiO4 is not thermodynamically stable. Its cell parameters are approximately a = 5.7 Å, b = 11.71 Å and c = 8.24 Å.

Iddingsite is a microcrystalline rock that is derived from alteration of olivine. It is usually studied as a mineral, and consists of a mixture of remnant olivine, clay minerals, iron oxides, and ferrihydrites. Debates over iddingsite's non-definite crystal structure caused it to be de-listed as an official mineral by the IMA; thus, it is properly referred to as a rock.
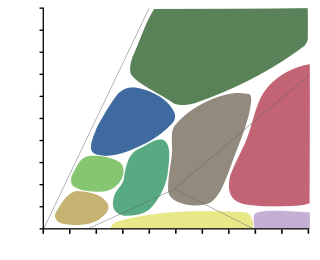
A metamorphic facies is a set of mineral assemblages in metamorphic rocks formed under similar pressures and temperatures. The assemblage is typical of what is formed in conditions corresponding to an area on the two dimensional graph of temperature vs. pressure. Rocks which contain certain minerals can therefore be linked to certain tectonic settings, times and places in the geological history of the area. The boundaries between facies are wide because they are gradational and approximate. The area on the graph corresponding to rock formation at the lowest values of temperature and pressure is the range of formation of sedimentary rocks, as opposed to metamorphic rocks, in a process called diagenesis.

Yuksporite is a rare inosilicate mineral with double width, unbranched chains, and the complicated chemical formula K4(Ca,Na)14Sr2Mn(Ti,Nb)4(O,OH)4(Si6O17)2(Si2O7)3(H2O,OH)3. It contains the relatively rare elements strontium, titanium and niobium, as well as the commoner metallic elements potassium, calcium, sodium and manganese. As with all silicates, it contains groups of linked silicon and oxygen atoms, as well as some associated water molecules.
Coyoteite is a hydrated sodium iron sulfide mineral. The mineral was named coyoteite after Coyote Peak near Orick, California, where it was discovered.

Cervandonite is a rare arsenosilicate mineral. It has a chemical formula (Ce,Nd,La)(Fe3+
,Fe2+
,Ti4+
,Al)
3SiAs(Si,As)O
13 or (Ce,Nd,La)(Fe3+
,Fe2+
,Ti,Al)
3O
2(Si
2O
7)(As3+
O
3)(OH). It has a monoclinic crustal structure with supercell (Z=6), the crystal structure was established as a trigonal subcell, with space group R3m and a = 6.508(1)Ǻ, c = 18.520(3) Ǻ, V 679.4(2) Ǻ3, and Z=3. It was first described by Buhler Armbruster in 1988, but it has proven to be problem due to the extreme scarcity of single crystals and its unusual replacement of silicon and arsenic. Cervandonite is named after the location where it was first described, Pizzo Cervandone (Scherbadung), Italy in the Central Alps.

Chamosite is the Fe2+end member of the chlorite group. A hydrous aluminium silicate of iron, which is produced in an environment of low to moderate grade of metamorphosed iron deposits, as gray or black crystals in oolitic iron ore. Like other chlorites, it is a product of the hydrothermal alteration of pyroxenes, amphiboles and biotite in igneous rock. The composition of chlorite is often related to that of the original igneous mineral so that more Fe-rich chlorites are commonly found as replacements of the Fe-rich ferromagnesian minerals (Deer et al., 1992).

Fluor-liddicoatite is a rare member of the tourmaline group of minerals, elbaite subgroup, and the theoretical calcium endmember of the elbaite-fluor-liddicoatite series; the pure end-member has not yet been found in nature. Fluor-liddicoatite is indistinguishable from elbaite by X-ray diffraction techniques. It forms a series with elbaite and probably also with olenite. Liddiocoatite is currently a non-approved mineral name, but Aurisicchio et al. (1999) and Breaks et al. (2008) found OH-dominant species. Formulae are
Paulscherrerite, UO2(OH)2, is a newly named mineral of the schoepite subgroup of hexavalent uranium hydrate/hydroxides. It is monoclinic, but no space group has been determined because no single-crystal study has been done. Paulscherrerite occurs as a canary yellow microcrystalline powdery product with a length of ~500 nm. It forms by the weathering and ultimate pseudomorphism of uranium-lead bearing minerals such as metaschoepite. The type locality for paulscherrerite is the Number 2 Workings, Radium Ridge near Mount Painter, North Flinders Ranges, South Australia, an area where radiogenic heat has driven hydrothermal activity for millions of years. It is named for Swiss physicist Paul Scherrer, co-inventor of the Debye-Scherrer X-ray powder diffraction camera. Study of paulscherrerite and related minerals is important for understanding the mobility of uranium around mining sites, as well as designing successful strategies for the storage of nuclear weapons and the containment of nuclear waste.

Bicchulite has an ideal chemical formula of 2CaO•Al2O2•SiO2•H2O, which was formularized from the hydrothermal synthesis of synthetic gehlenite. Also, bicchulite was sighted in the mines of Japan with related minerals. This sodalite-type structured bicchulite has an uncommon ratio of aluminium to silicon, causing difficulties deciphering the structure. Because of bicchulite's structure it has a powdery texture, which leads to complications in obtaining information on the mineral's physical properties. Despite this problem, the color, specific gravity, and crystal size of bicchulite are known. Although bicchulite was only discovered about 40 years ago, technology has been rapidly advancing, allowing more accurate results to be made from experiments done today.

Tumchaite, Na2(Zr,Sn)Si4O11·H2O, is a colorless to white monoclinic phyllosilicate mineral. It is associated with calcite, dolomite, and pyrite in the late dolomite-calcite carbonatites. It can be transparent to translucent; has a vitreous luster; and has perfect cleavage on {100}. Its hardness is 4.5, between fluorite and apatite. Tumchaite is isotypic with penkvilksite. The structure of the mineral is identified by silicate sheets parallel {100}, formed by alternation of clockwise and counterclockwise growing spiral chains of corner-sharing SiO4 tetrahedra. Tumchaite is named for the river Tumcha near Vuoriyarvi massif.
George-ericksenite is a mineral with the chemical formula Na6CaMg(IO3)6(CrO4)2(H2O)12. It is vitreous, pale yellow to bright lemon yellow, brittle, and features a prismatic to acicular crystal habit along [001] and somewhat flattened crystal habit on {110}. It was first encountered in 1984 at the Pinch Mineralogical Museum. One specimen of dietzeite from Oficina Chacabuco, Chile had bright lemon-yellow micronodules on it. These crystals produced an X-ray powder diffraction pattern that did not match any XRD data listed for inorganic compounds. The X-ray diffraction pattern and powder mount were set aside until 1994. By then, the entire mineral collection from the Pinch Mineralogical Museum had been purchased by the Canadian Museum of Nature. The specimen was then retrieved and studied further. This study was successful and the new mineral george-ericksenite was discovered. The mineral was named for George E. Ericksen who was a research economic geologist with the U.S. Geological Survey for fifty years. The mineral and name have been approved by Commission on New Minerals and Mineral Names (IMA). The specimen, polished thin section, and the actual crystal used for the structure determination are kept in the Display Series of the National Mineral Collection of Canada at the Canadian Museum of Nature, Ottawa, Ontario.
Alloriite is a silicate mineral that belongs to the cancrinite group, or more specifically the feldspathoid group. It is currently only found in Italy. It was discovered by and named for the Italian mineralogist Roberto Allori, an avid mineral collector who has also done research on piergorite and willhendersonite. The mineral appears as a crystal that is approximately 1.5 by 2mm in length. The crystal grows as both tabular and prismatic crystals, and commonly occurs with sanidine, biotite, andradite, and apatite. It was approved of being a mineral in 2006 by the International Mineralogical Association. Afghanite is a cancrinite group mineral that is very similar to alloriite in both its chemical composition and its physical properties.
Yangite (PbMnSi3O8•H2O) is a chain-silicate mineral, first discovered within the Kombat mine in Namibia. The mineral is named after Hexiong Yang, a researcher at the University of Arizona's Department of Geosciences. Yangite was approved as a valid mineral species by the International Mineralogical Association in 2012.
Rayite, a monoclinic mineral containing Lead-Silver-Thallium-Antimony, was found during microscopic and electron microprobe study of specimens from the complex, polymetallic sulphide-native metal sulpho-salt paragenesis of Rajpura-Dariba, Rajasthan, India. It is named after Dr. Santosh K. Ray of President College, Calcutta, India. It bears a striking resemblance to owyheeite in terms of its Lead/(Silver,Thallium)/Antimony ratio, yet its structural affinity lies with Semseyite. The average composition is Lead-47.06, Copper-0.03, Silver-4.54, Thallium-2.04, Antimony-27.42, Sulphur-19.59 by wt.% (total 100.68) suggesting an ideal formula of Pb8(Ag,Tl)2Sb8S21, where Ag > Tl. Meneghinite, Owyheeite, and Galena are related minerals.
References
- ↑ Warr, L.N. (2021). "IMA–CNMNC approved mineral symbols". Mineralogical Magazine. 85 (3): 291–320. Bibcode:2021MinM...85..291W. doi: 10.1180/mgm.2021.43 . S2CID 235729616.
- 1 2 3 "Khmaralite". mindat.org. Hudson Institute of Mineralogy. Retrieved 2015-11-23.
- 1 2 3 Barthelmy, David. "Khmaralite Mineral Data". webmineral.com. David Barthelmy. Retrieved 2015-12-01.
- 1 2 3 4 5 6 7 8 9 10 11 12 Barbier, Jacques; Grew, Edward S.; Moore, Paulus B.; Su, Shu-Chun (1999). "Khmaralite, a new beryllium-bearing mineral related to sapphirine: A superstructure resulting from partial ordering of Be, Al, and Si on tetrahedral sites" (PDF). American Mineralogist. 84 (American Mineralogist): 1650–1660. Bibcode:1999AmMin..84.1650B. doi:10.2138/am-1999-1020. S2CID 53383954 . Retrieved 2015-12-01.