
Polystyrene (PS) is a synthetic polymer made from monomers of the aromatic hydrocarbon styrene. Polystyrene can be solid or foamed. General-purpose polystyrene is clear, hard, and brittle. It is an inexpensive resin per unit weight. It is a poor barrier to air and water vapor and has a relatively low melting point. Polystyrene is one of the most widely used plastics, with the scale of its production being several million tonnes per year. Polystyrene is naturally transparent, but can be colored with colorants. Uses include protective packaging, containers, lids, bottles, trays, tumblers, disposable cutlery, in the making of models, and as an alternative material for phonograph records.

A thermoplastic, or thermosoftening plastic, is any plastic polymer material that becomes pliable or moldable at a certain elevated temperature and solidifies upon cooling.
In polymer chemistry, living polymerization is a form of chain growth polymerization where the ability of a growing polymer chain to terminate has been removed. This can be accomplished in a variety of ways. Chain termination and chain transfer reactions are absent and the rate of chain initiation is also much larger than the rate of chain propagation. The result is that the polymer chains grow at a more constant rate than seen in traditional chain polymerization and their lengths remain very similar. Living polymerization is a popular method for synthesizing block copolymers since the polymer can be synthesized in stages, each stage containing a different monomer. Additional advantages are predetermined molar mass and control over end-groups.
In polymer chemistry, an addition polymer is a polymer that forms by simple linking of monomers without the co-generation of other products. Addition polymerization differs from condensation polymerization, which does co-generate a product, usually water. Addition polymers can be formed by chain polymerization, when the polymer is formed by the sequential addition of monomer units to an active site in a chain reaction, or by polyaddition, when the polymer is formed by addition reactions between species of all degrees of polymerization. Addition polymers are formed by the addition of some simple monomer units repeatedly. Generally polymers are unsaturated compounds like alkenes, alkalines etc. The addition polymerization mainly takes place in free radical mechanism. The free radical mechanism of addition polymerization completed by three steps i.e. Initiation of free radical, Chain propagation, Termination of chain.

Polymer degradation is the reduction in the physical properties of a polymer, such as strength, caused by changes in its chemical composition. Polymers and particularly plastics are subject to degradation at all stages of their product life cycle, including during their initial processing, use, disposal into the environment and recycling. The rate of this degradation varies significantly; biodegradation can take decades, whereas some industrial processes can completely decompose a polymer in hours.

In polymer chemistry, a copolymer is a polymer derived from more than one species of monomer. The polymerization of monomers into copolymers is called copolymerization. Copolymers obtained from the copolymerization of two monomer species are sometimes called bipolymers. Those obtained from three and four monomers are called terpolymers and quaterpolymers, respectively. Copolymers can be characterized by a variety of techniques such as NMR spectroscopy and size-exclusion chromatography to determine the molecular size, weight, properties, and composition of the material.
In polymer chemistry, anionic addition polymerization is a form of chain-growth polymerization or addition polymerization that involves the polymerization of monomers initiated with anions. The type of reaction has many manifestations, but traditionally vinyl monomers are used. Often anionic polymerization involves living polymerizations, which allows control of structure and composition.
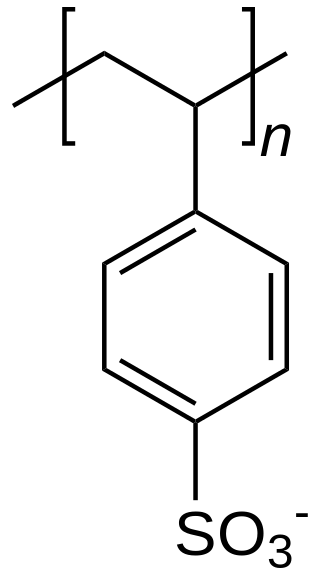
Polystyrene sulfonates are a group of medications used to treat high blood potassium. Effects generally take hours to days. They are also used to remove potassium, calcium, and sodium from solutions in technical applications.
In polymer chemistry, vinyl polymers are a group of polymers derived from substituted vinyl monomers. Their backbone is an extended alkane chain [−CH2−CHR−]. In popular usage, "vinyl" refers only to polyvinyl chloride (PVC).
Thermoplastic elastomers (TPE), sometimes referred to as thermoplastic rubbers (TPR), are a class of copolymers or a physical mix of polymers that consist of materials with both thermoplastic and elastomeric properties.
Solution polymerization is a method of industrial polymerization. In this procedure, a monomer is dissolved in a non-reactive solvent that contains a catalyst or initiator.
Rubber toughening is a process in which rubber nanoparticles are interspersed within a polymer matrix to increase the mechanical robustness, or toughness, of the material. By "toughening" a polymer it is meant that the ability of the polymeric substance to absorb energy and plastically deform without fracture is increased. Considering the significant advantages in mechanical properties that rubber toughening offers, most major thermoplastics are available in rubber-toughened versions; for many engineering applications, material toughness is a deciding factor in final material selection.

Willis Harmon Ray is an American chemical engineer, control theorist, applied mathematician, and a Vilas Research emeritus professor at the University of Wisconsin–Madison notable for being the 2000 winner of the prestigious Richard E. Bellman Control Heritage Award and the 2019 winner of the Neal Amundson Award.

In polymer chemistry photo-oxidation is the degradation of a polymer surface due to the combined action of light and oxygen. It is the most significant factor in the weathering of plastics. Photo-oxidation causes the polymer chains to break, resulting in the material becoming increasingly brittle. This leads to mechanical failure and, at an advanced stage, the formation of microplastics. In textiles the process is called phototendering.
Macromolecular Reaction Engineering is a peer-reviewed scientific journal published monthly by Wiley-VCH. The journal covers academic and industrial research in the field of polymer reaction engineering, which includes polymer science. It emerged from a section that was part of Macromolecular Materials and Engineering. The journal publishes reviews, feature articles, communications, and full papers in the entire field of polymer reaction engineering, including polymer reaction modeling, reactor optimization, and control. Its 2020 impact factor is 1.931.

Living free radical polymerization is a type of living polymerization where the active polymer chain end is a free radical. Several methods exist. IUPAC recommends to use the term "reversible-deactivation radical polymerization" instead of "living free radical polymerization", though the two terms are not synonymous.

In polymer chemistry, graft polymers are segmented copolymers with a linear backbone of one composite and randomly distributed branches of another composite. The picture labeled "graft polymer" shows how grafted chains of species B are covalently bonded to polymer species A. Although the side chains are structurally distinct from the main chain, the individual grafted chains may be homopolymers or copolymers. Graft polymers have been synthesized for many decades and are especially used as impact resistant materials, thermoplastic elastomers, compatibilizers, or emulsifiers for the preparation of stable blends or alloys. One of the better-known examples of a graft polymer is a component used in high impact polystyrene, consisting of a polystyrene backbone with polybutadiene grafted chains.

Acrylonitrile styrene acrylate (ASA), also called acrylic styrene acrylonitrile, is an amorphous thermoplastic developed as an alternative to acrylonitrile butadiene styrene (ABS), that has improved weather resistance. It is an acrylate rubber-modified styrene acrylonitrile copolymer. It is used for general prototyping in 3D printing, where its UV resistance and mechanical properties make it an excellent material for use in fused filament fabrication printers, particularly for outdoor applications. ASA is also widely used in the automotive industry.

Automated synthesis or automatic synthesis is a set of techniques that use robotic equipment to perform chemical synthesis in an automated way. Automating processes allows for higher efficiency and product quality although automation technology can be cost-prohibitive and there are concerns regarding overdependence and job displacement. Chemical processes were automated throughout the 19th and 20th centuries, with major developments happening in the previous thirty years, as technology advanced. Tasks that are performed may include: synthesis in variety of different conditions, sample preparation, purification, and extractions. Applications of automated synthesis are found on research and industrial scales in a wide variety of fields including polymers, personal care, and radiosynthesis.
Brigitte Voit is a German chemist and professor of chemistry. She holds the chair Organic Chemistry of Polymers at the Faculty of Chemistry of the TU Dresden and is head of the Institute of Macromolecular Chemistry at the Leibniz Institute of Polymer Research in Dresden. From September 1, 2002, to July 31, 2022, she was also member of the Board of Management/CSO of the IPF Dresden.










