
A tumor suppressor gene (TSG), or anti-oncogene, is a gene that regulates a cell during cell division and replication. If the cell grows uncontrollably, it will result in cancer. When a tumor suppressor gene is mutated, it results in a loss or reduction in its function. In combination with other genetic mutations, this could allow the cell to grow abnormally. The loss of function for these genes may be even more significant in the development of human cancers, compared to the activation of oncogenes.

Renato Dulbecco was an Italian–American virologist who won the 1975 Nobel Prize in Physiology or Medicine for his work on oncoviruses, which are viruses that can cause cancer when they infect animal cells. He studied at the University of Turin under Giuseppe Levi, along with fellow students Salvador Luria and Rita Levi-Montalcini, who also moved to the U.S. with him and won Nobel prizes. He was drafted into the Italian army in World War II, but later joined the resistance.

A neoplasm is a type of abnormal and excessive growth of tissue. The process that occurs to form or produce a neoplasm is called neoplasia. The growth of a neoplasm is uncoordinated with that of the normal surrounding tissue, and persists in growing abnormally, even if the original trigger is removed. This abnormal growth usually forms a mass, when it may be called a tumour or tumor.
The Knudson hypothesis, also known as the two-hit hypothesis, is the hypothesis that most tumor suppressor genes require both alleles to be inactivated, either through mutations or through epigenetic silencing, to cause a phenotypic change. It was first formulated by Alfred G. Knudson in 1971 and led indirectly to the identification of tumor suppressor genes. Knudson won the 1998 Albert Lasker Clinical Medical Research Award for this work.

Alfred George Knudson, Jr. was an American physician and geneticist specializing in cancer genetics. Among his many contributions to the field was the formulation of the Knudson hypothesis in 1971, which explains the effects of mutation on carcinogenesis.
Carcinogenesis, also called oncogenesis or tumorigenesis, is the formation of a cancer, whereby normal cells are transformed into cancer cells. The process is characterized by changes at the cellular, genetic, and epigenetic levels and abnormal cell division. Cell division is a physiological process that occurs in almost all tissues and under a variety of circumstances. Normally, the balance between proliferation and programmed cell death, in the form of apoptosis, is maintained to ensure the integrity of tissues and organs. According to the prevailing accepted theory of carcinogenesis, the somatic mutation theory, mutations in DNA and epimutations that lead to cancer disrupt these orderly processes by interfering with the programming regulating the processes, upsetting the normal balance between proliferation and cell death. This results in uncontrolled cell division and the evolution of those cells by natural selection in the body. Only certain mutations lead to cancer whereas the majority of mutations do not.

DNA mismatch repair (MMR) is a system for recognizing and repairing erroneous insertion, deletion, and mis-incorporation of bases that can arise during DNA replication and recombination, as well as repairing some forms of DNA damage.

Bert Vogelstein is director of the Ludwig Center, Clayton Professor of Oncology and Pathology and a Howard Hughes Medical Institute investigator at The Johns Hopkins Medical School and Sidney Kimmel Comprehensive Cancer Center. A pioneer in the field of cancer genomics, his studies on colorectal cancers revealed that they result from the sequential accumulation of mutations in oncogenes and tumor suppressor genes. These studies now form the paradigm for modern cancer research and provided the basis for the notion of the somatic evolution of cancer.

Mary-Claire King is an American geneticist. She was the first to show that breast cancer can be inherited due to mutations in the gene she called BRCA1. She studies human genetics and is particularly interested in genetic heterogeneity and complex traits. She studies the interaction of genetics and environmental influences and their effects on human conditions such as breast and ovarian cancer, inherited deafness, schizophrenia, HIV, systemic lupus erythematosus and rheumatoid arthritis. She has been the American Cancer Society Professor of the Department of Genome Sciences and of Medical Genetics in the Department of Medicine at the University of Washington since 1995.
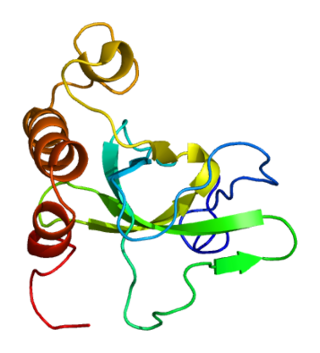
MSH6 or mutS homolog 6 is a gene that codes for DNA mismatch repair protein Msh6 in the budding yeast Saccharomyces cerevisiae. It is the homologue of the human "G/T binding protein," (GTBP) also called p160 or hMSH6. The MSH6 protein is a member of the Mutator S (MutS) family of proteins that are involved in DNA damage repair.

Victor E. Velculescu is a Professor of Oncology and Co-Director of Cancer Biology at Johns Hopkins University School of Medicine. He is internationally known for his discoveries in genomics and cancer research.

DNA polymerase beta, also known as POLB, is an enzyme present in eukaryotes. In humans, it is encoded by the POLB gene.

Cyclin-dependent kinase inhibitor 1C , also known as CDKN1C, is a protein which in humans is encoded by the CDKN1C imprinted gene.
Somatic evolution is the accumulation of mutations and epimutations in somatic cells during a lifetime, and the effects of those mutations and epimutations on the fitness of those cells. This evolutionary process has first been shown by the studies of Bert Vogelstein in colon cancer. Somatic evolution is important in the process of aging as well as the development of some diseases, including cancer.
Cancer cells are cells that divide continually, forming solid tumors or flooding the blood or lymph with abnormal cells. Cell division is a normal process used by the body for growth and repair. A parent cell divides to form two daughter cells, and these daughter cells are used to build new tissue or to replace cells that have died because of aging or damage. Healthy cells stop dividing when there is no longer a need for more daughter cells, but cancer cells continue to produce copies. They are also able to spread from one part of the body to another in a process known as metastasis.
Chromosomal instability (CIN) is a type of genomic instability in which chromosomes are unstable, such that either whole chromosomes or parts of chromosomes are duplicated or deleted. More specifically, CIN refers to the increase in rate of addition or loss of entire chromosomes or sections of them. The unequal distribution of DNA to daughter cells upon mitosis results in a failure to maintain euploidy leading to aneuploidy. In other words, the daughter cells do not have the same number of chromosomes as the cell they originated from. Chromosomal instability is the most common form of genetic instability and cause of aneuploidy.

Circulating tumor DNA (ctDNA) is tumor-derived fragmented DNA in the bloodstream that is not associated with cells. ctDNA should not be confused with cell-free DNA (cfDNA), a broader term which describes DNA that is freely circulating in the bloodstream, but is not necessarily of tumor origin. Because ctDNA may reflect the entire tumor genome, it has gained traction for its potential clinical utility; "liquid biopsies" in the form of blood draws may be taken at various time points to monitor tumor progression throughout the treatment regimen.

William G. Kaelin Jr. is an American Nobel laureate physician-scientist. He is a professor of medicine at Harvard University and the Dana–Farber Cancer Institute. His laboratory studies tumor suppressor proteins. In 2016, Kaelin received the Albert Lasker Award for Basic Medical Research and the AACR Princess Takamatsu Award. He also won the Nobel Prize in Physiology or Medicine in 2019 along with Peter J. Ratcliffe and Gregg L. Semenza.

Scott William Lowe is Chair of the Cancer Biology and Genetics Program in the Sloan Kettering Institute at Memorial Sloan Kettering Cancer Center. He is recognized for his research on the tumor suppressor gene, p53, which is mutated in nearly half of cancers.

Curtis. C. Harris is the head of the Molecular Genetics and Carcinogenesis Section and chief of the Laboratory of Human Carcinogenesis at the Center for Cancer Research of the National Cancer Institute, NIH.
















