Related Research Articles

A reverse transcriptase (RT) is an enzyme used to convert RNA genome to DNA, a process termed reverse transcription. Reverse transcriptases are used by viruses such as HIV and hepatitis B to replicate their genomes, by retrotransposon mobile genetic elements to proliferate within the host genome, and by eukaryotic cells to extend the telomeres at the ends of their linear chromosomes. Contrary to a widely held belief, the process does not violate the flows of genetic information as described by the classical central dogma, as transfers of information from RNA to DNA are explicitly held possible.

A DNA polymerase is a member of a family of enzymes that catalyze the synthesis of DNA molecules from nucleoside triphosphates, the molecular precursors of DNA. These enzymes are essential for DNA replication and usually work in groups to create two identical DNA duplexes from a single original DNA duplex. During this process, DNA polymerase "reads" the existing DNA strands to create two new strands that match the existing ones. These enzymes catalyze the chemical reaction
The Stanford University School of Medicine is the medical school of Stanford University and is located in Stanford, California, United States. It traces its roots to the Medical Department of the University of the Pacific, founded in San Francisco in 1858. This medical institution, then called Cooper Medical College, was acquired by Stanford in 1908. The medical school moved to the Stanford campus near Palo Alto, California, in 1959.
DNA gyrase, or simply gyrase, is an enzyme within the class of topoisomerase and is a subclass of Type II topoisomerases that reduces topological strain in an ATP dependent manner while double-stranded DNA is being unwound by elongating RNA-polymerase or by helicase in front of the progressing replication fork. It is the only known enzyme to actively contribute negative supercoiling to DNA, while it also is capable of relaxing positive supercoils. It does so by looping the template to form a crossing, then cutting one of the double helices and passing the other through it before releasing the break, changing the linking number by two in each enzymatic step. This process occurs in bacteria, whose single circular DNA is cut by DNA gyrase and the two ends are then twisted around each other to form supercoils. Gyrase is also found in eukaryotic plastids: it has been found in the apicoplast of the malarial parasite Plasmodium falciparum and in chloroplasts of several plants. Bacterial DNA gyrase is the target of many antibiotics, including nalidixic acid, novobiocin, albicidin, and ciprofloxacin.

Eukaryotic DNA replication is a conserved mechanism that restricts DNA replication to once per cell cycle. Eukaryotic DNA replication of chromosomal DNA is central for the duplication of a cell and is necessary for the maintenance of the eukaryotic genome.
DNA polymerase iota is an enzyme that in humans is encoded by the POLI gene. It is found in higher eukaryotes, and is believed to have arisen from a gene duplication from Pol η. Pol ι, is a Y family polymerase that is involved in translesion synthesis. It can bypass 6-4 pyrimidine adducts and abasic sites and has a high frequency of wrong base incorporation. Like many other Y family polymerases Pol ι, has low processivity, a large DNA binding pocket and doesn't undergo conformational changes when DNA binds. These attributes are what allow Pol ι to carry out its task as a translesion polymerase. Pol ι only uses Hoogsteen base pairing, during DNA synthesis, it will add adenine opposite to thymine in the syn conformation and can add both cytosine and thymine in the anti conformation across guanine, which it flips to the syn conformation.
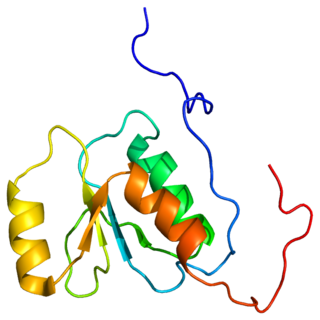
Replication factor C subunit 1 is a protein that in humans is encoded by the RFC1 gene.

Replication factor C subunit 4 is a protein that in humans is encoded by the RFC4 gene.

Replication factor C subunit 2 is a protein that in humans is encoded by the RFC2 gene.

Replication factor C subunit 3 is a protein that in humans is encoded by the RFC3 gene.

Replication factor C subunit 5 is a protein that in humans is encoded by the RFC5 gene.

DNA polymerase theta is an enzyme that in humans is encoded by the POLQ gene. This polymerase plays a key role in one of the three major double strand break repair pathways: theta-mediated end joining (TMEJ). Most double-strand breaks are repaired by non-homologous end joining (NHEJ) or homology directed repair (HDR). However, in some contexts, NHEJ and HR are insufficient and TMEJ is the only solution to repair the break. TMEJ is often described as alternative NHEJ, but differs in that it lacks a requirement for the Ku heterodimer, and it can only act on resected DNA ends. Following annealing of short regions on the DNA overhangs, DNA polymerase theta catalyzes template-dependent DNA synthesis across the broken ends, stabilizing the paired structure.

Bruce William Stillman is a biochemist and cancer researcher who has served as the Director of Cold Spring Harbor Laboratory (CSHL) since 1994 and President since 2003. He also served as the Director of its NCI-designated Cancer Center for 25 years from 1992 to 2016. During his leadership, CSHL has been ranked as the No. 1 institution in molecular biology and genetics research by Thomson Reuters. Stillman's research focuses on how chromosomes are duplicated in human cells and in yeast Saccharomyces cerevisiae; the mechanisms that ensure accurate inheritance of genetic material from one generation to the next; and how missteps in this process lead to cancer. For his accomplishments, Stillman has received numerous awards, including the Alfred P. Sloan, Jr. Prize in 2004 and the 2010 Louisa Gross Horwitz Prize, both of which he shared with Thomas J. Kelly of Memorial Sloan-Kettering Cancer Center, as well as the 2019 Canada Gairdner International Award for biomedical research, which he shared with John Diffley.
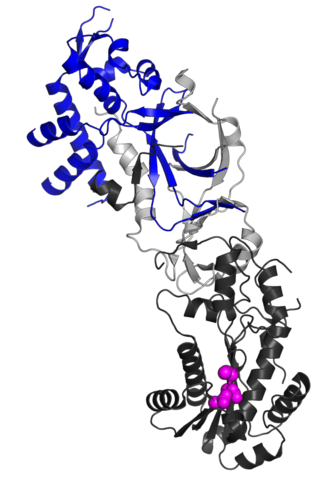
Ribonuclease H2, subunit B is a protein in humans is encoded by the RNASEH2B gene. RNase H2 is composed of a single catalytic subunit (A) and two non-catalytic subunits, and degrades the RNA of RNA:DNA hybrids. The non-catalytic B subunit of RNase H2 is thought to play a role in DNA replication.
The minimal genome is a concept which can be defined as the set of genes sufficient for life to exist and propagate under nutrient-rich and stress-free conditions. Alternatively, it can also be defined as the gene set supporting life on an axenic cell culture in rich media, and it is thought what makes up the minimal genome will depend on the environmental conditions that the organism inhabits. By one early investigation, the minimal genome of a bacterium should include a virtually complete set of proteins for replication and translation, a transcription apparatus including four subunits of RNA polymerase including the sigma factor rudimentary proteins sufficient for recombination and repair, several chaperone proteins, the capacity for anaerobic metabolism through glycolysis and substrate-level phosphorylation, transamination of glutamyl-tRNA to glutaminyl-tRNA, lipid biosynthesis, eight cofactor enzymes, protein export machinery, and a limited metabolite transport network including membrane ATPases. Proteins involved in the minimum bacterial genome tend to be substantially more related to proteins found in archaea and eukaryotes compared to the average gene in the bacterial genome more generally indicating a substantial number of universally conserved proteins. The minimal genomes reconstructed on the basis of existing genes does not preclude simpler systems in more primitive cells, such as an RNA world genome which does not have the need for DNA replication machinery, which is otherwise part of the minimal genome of current cells.
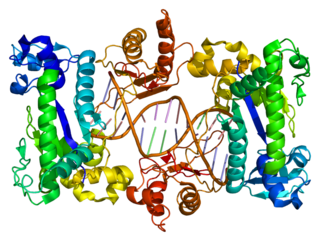
DNA Polymerase V is a polymerase enzyme involved in DNA repair mechanisms in bacteria, such as Escherichia coli. It is composed of a UmuD' homodimer and a UmuC monomer, forming the UmuD'2C protein complex. It is part of the Y-family of DNA Polymerases, which are capable of performing DNA translesion synthesis (TLS). Translesion polymerases bypass DNA damage lesions during DNA replication - if a lesion is not repaired or bypassed the replication fork can stall and lead to cell death. However, Y polymerases have low sequence fidelity during replication. When the UmuC and UmuD' proteins were initially discovered in E. coli, they were thought to be agents that inhibit faithful DNA replication and caused DNA synthesis to have high mutation rates after exposure to UV-light. The polymerase function of Pol V was not discovered until the late 1990s when UmuC was successfully extracted, consequent experiments unequivocally proved UmuD'2C is a polymerase. This finding lead to the detection of many Pol V orthologs and the discovery of the Y-family of polymerases.

Lucy Shapiro is an American developmental biologist. She is a professor of Developmental Biology at the Stanford University School of Medicine. She is the Virginia and D.K. Ludwig Professor of Cancer Research and the director of the Beckman Center for Molecular and Genetic Medicine.

DNA polymerase alpha catalytic subunit is an enzyme that in humans is encoded by the POLA1 gene.
Mycoplasma orale is a small bacterium found in the class Mollicutes. It belongs to the genus Mycoplasma, a well-known group of bacterial parasites that inhabit humans. It also is known to be an opportunistic pathogen in immunocompromised humans. As with other Mycoplasma species, M. orale is not readily treated with many antibiotics due to its lack of a peptidoglycan cell wall. Therefore, this species is relevant to the medical field as physicians face the task of treating patients infected with this microbe. It is characterized by a small physical size, a small genome size, and a limited metabolism. It is also known to frequently contaminate laboratory experiments. This bacteria is very similar physiologically and morphologically to its sister species within the genus Mycoplasma; however, its recent discovery leaves many questions still unanswered about this microbe.
Teresa Shu-Fong Wang is an American biochemist. She is a professor emeritus at Stanford University, and the K. Bensch Endowed Chair Professor in Experimental Pathology of Department of Pathology at the Stanford University School of Medicine. Her scientific pursuit focuses on the biochemical mechanisms of chromosome replication proteins, and molecular mechanisms of their involvement in maintaining genome integrity during chromosome replication.
References
- ↑ "Linda Minium Boxer '73". Carleton College. Retrieved 2024-01-20.
- 1 2 3 "Linda Boxer, MD, PhD's Profile". Stanford Profiles. Retrieved 2024-01-20.
- 1 2 Boxer, Linda Minium (1981). Biochemical Studies of DNA Replication: I. A DNA polymerase from Mycoplasma Orale: II. A DNA-dependent Atpase from KB Cells (Ph.D. thesis). Stanford University. OCLC 38637156.
- 1 2 "Dr. Linda Boxer Vice Dean". School of Medicine (in Samoan). Retrieved 2024-01-20.