

Arnold Sommerfeld was a German theoretical physicist whom the following is named after:

In atomic physics, the Bohr model or Rutherford–Bohr model was the first successful model of the atom. Developed from 1911 to 1918 by Niels Bohr and building on Ernest Rutherford's nuclear model, it supplanted the plum pudding model of J J Thomson only to be replaced by the quantum atomic model in the 1920s. It consists of a small, dense nucleus surrounded by orbiting electrons. It is analogous to the structure of the Solar System, but with attraction provided by electrostatic force rather than gravity, and with the electron energies quantized.

In physics, a surface wave is a mechanical wave that propagates along the interface between differing media. A common example is gravity waves along the surface of liquids, such as ocean waves. Gravity waves can also occur within liquids, at the interface between two fluids with different densities. Elastic surface waves can travel along the surface of solids, such as Rayleigh or Love waves. Electromagnetic waves can also propagate as "surface waves" in that they can be guided along with a refractive index gradient or along an interface between two media having different dielectric constants. In radio transmission, a ground wave is a guided wave that propagates close to the surface of the Earth.
In physics, a correspondence principle is any one of several premises or assertions about the relationship between classical and quantum mechanics. The physicist Niels Bohr coined the term in 1920 during the early development of quantum theory; he used it to explain how quantized classical orbitals connect to quantum radiation. Modern sources often use the term for the idea that the behavior of systems described by quantum theory reproduces classical physics in the limit of large quantum numbers: for large orbits and for large energies, quantum calculations must agree with classical calculations. A "generalized" correspondence principle refers to the requirement for a broad set of connections between any old and new theory.
Solid-state physics is the study of rigid matter, or solids, through methods such as solid-state chemistry, quantum mechanics, crystallography, electromagnetism, and metallurgy. It is the largest branch of condensed matter physics. Solid-state physics studies how the large-scale properties of solid materials result from their atomic-scale properties. Thus, solid-state physics forms a theoretical basis of materials science. Along with solid-state chemistry, it also has direct applications in the technology of transistors and semiconductors.

Arnold Johannes Wilhelm Sommerfeld, was a German theoretical physicist who pioneered developments in atomic and quantum physics, and also educated and mentored many students for the new era of theoretical physics. He served as doctoral supervisor and postdoc supervisor to seven Nobel Prize winners and supervised at least 30 other famous physicists and chemists. Only J. J. Thomson's record of mentorship offers a comparable list of high-achieving students.

In quantum physics and chemistry, quantum numbers are quantities that characterize the possible states of the system. To fully specify the state of the electron in a hydrogen atom, four quantum numbers are needed. The traditional set of quantum numbers includes the principal, azimuthal, magnetic, and spin quantum numbers. To describe other systems, different quantum numbers are required. For subatomic particles, one needs to introduce new quantum numbers, such as the flavour of quarks, which have no classical correspondence.
Paul Peter Ewald, FRS was a German crystallographer and physicist, a pioneer of X-ray diffraction methods.
In solid-state physics, the free electron model is a quantum mechanical model for the behaviour of charge carriers in a metallic solid. It was developed in 1927, principally by Arnold Sommerfeld, who combined the classical Drude model with quantum mechanical Fermi–Dirac statistics and hence it is also known as the Drude–Sommerfeld model.
The old quantum theory is a collection of results from the years 1900–1925 which predate modern quantum mechanics. The theory was never complete or self-consistent, but was instead a set of heuristic corrections to classical mechanics. The theory has come to be understood as the semi-classical approximation to modern quantum mechanics. The main and final accomplishments of the old quantum theory were the determination of the modern form of the periodic table by Edmund Stoner and the Pauli exclusion principle which were both premised on the Arnold Sommerfeld enhancements to the Bohr model of the atom.
Wilhelm Lenz was a German physicist, most notable for his invention of the Ising model, and for his application of the Laplace–Runge–Lenz vector to the old quantum mechanical treatment of hydrogen-like atoms.

Walther Ludwig Julius Kossel was a German physicist known for his theory of the chemical bond, Sommerfeld–Kossel displacement law of atomic spectra, the Kossel-Stranski model for crystal growth, and the Kossel effect. Walther was the son of Albrecht Kossel who won the Nobel Prize in Physiology or Medicine in 1910.
B. Adolf Kratzer was a German theoretical physicist who made contributions to atomic physics and molecular physics, and was an authority on molecular band spectroscopy. He was born in Günzburg and died in Münster.

Helmut Hönl was a German theoretical physicist who made contributions to quantum mechanics and the understanding of atomic and molecular structure.
Karl Richard Bechert was a German theoretical physicist and political leader. As a scientist, he made contributions in atomic physics.

William Vermillion Houston was an American physicist who made contributions to spectroscopy, quantum mechanics, and solid-state physics as well as being a teacher and administrator. He became the second president of Rice University in 1946.
In chemistry and atomic physics, an electron shell may be thought of as an orbit that electrons follow around an atom's nucleus. The closest shell to the nucleus is called the "1 shell" (also called the "K shell"), followed by the "2 shell" (or "L shell"), then the "3 shell" (or "M shell"), and so on farther and farther from the nucleus. The shells correspond to the principal quantum numbers (n = 1, 2, 3, 4 ...) or are labeled alphabetically with the letters used in X-ray notation (K, L, M, ...). A useful guide when understanding electron shells in atoms is to note that each row on the conventional periodic table of elements represents an electron shell.
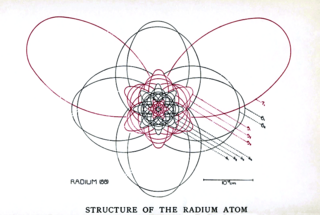
The Bohr–Sommerfeld model was an extension of the Bohr model to allow elliptical orbits of electrons around an atomic nucleus. Bohr–Sommerfeld theory is named after Danish physicist Niels Bohr and German physicist Arnold Sommerfeld. Sommerfeld argued that if electronic orbits could be elliptical instead of circular, the energy of the electron would be the same, except in the presence of a magnetic field, introducing what is now known as quantum degeneracy.
Jun Ishiwara or Atsushi Ishihara was a Japanese theoretical physicist, known for his works on the electronic theory of metals, the theory of relativity and quantum theory. Being the only Japanese scientist who made an original contribution to the old quantum theory, in 1915, independently of other scientists, he formulated quantization rules for systems with several degrees of freedom.
In mechanics, Sommerfeld effect is a phenomenon arising from feedback in the energy exchange between vibrating systems: for example, when for the rocking table, under given conditions, energy transmitted to the motor resulted not in higher revolutions but in stronger vibrations of the table. It is named after Arnold Sommerfeld. In 1902, A. Sommerfeld analyzed the vibrations caused by a motor driving an unbalanced weight and wrote that "This experiment corresponds roughly to the case in which a factory owner has a machine set on a poor foundation running at 30 horsepower. He achieves an effective level of just 1/3, however, because only 10 horsepower are doing useful work, while 20 horsepower are transferred to the foundational masonry". First mathematical descriptions of Sommerfeld effect were suggested by I. Blekhman and V. Konenko.
The Sommerfeld model can refer to: