
In physics, theBardeen–Cooper–Schrieffer (BCS) theory is the first microscopic theory of superconductivity since Heike Kamerlingh Onnes's 1911 discovery. The theory describes superconductivity as a microscopic effect caused by a condensation of Cooper pairs. The theory is also used in nuclear physics to describe the pairing interaction between nucleons in an atomic nucleus.

Condensed matter physics is the field of physics that deals with the macroscopic and microscopic physical properties of matter, especially the solid and liquid phases that arise from electromagnetic forces between atoms and electrons. More generally, the subject deals with condensed phases of matter: systems of many constituents with strong interactions among them. More exotic condensed phases include the superconducting phase exhibited by certain materials at extremely low cryogenic temperatures, the ferromagnetic and antiferromagnetic phases of spins on crystal lattices of atoms, the Bose–Einstein condensates found in ultracold atomic systems, and liquid crystals. Condensed matter physicists seek to understand the behavior of these phases by experiments to measure various material properties, and by applying the physical laws of quantum mechanics, electromagnetism, statistical mechanics, and other physics theories to develop mathematical models and predict the properties of extremely large groups of atoms.

Paramagnetism is a form of magnetism whereby some materials are weakly attracted by an externally applied magnetic field, and form internal, induced magnetic fields in the direction of the applied magnetic field. In contrast with this behavior, diamagnetic materials are repelled by magnetic fields and form induced magnetic fields in the direction opposite to that of the applied magnetic field. Paramagnetic materials include most chemical elements and some compounds; they have a relative magnetic permeability slightly greater than 1 and hence are attracted to magnetic fields. The magnetic moment induced by the applied field is linear in the field strength and rather weak. It typically requires a sensitive analytical balance to detect the effect and modern measurements on paramagnetic materials are often conducted with a SQUID magnetometer.

An ideal gas is a theoretical gas composed of many randomly moving point particles that are not subject to interparticle interactions. The ideal gas concept is useful because it obeys the ideal gas law, a simplified equation of state, and is amenable to analysis under statistical mechanics. The requirement of zero interaction can often be relaxed if, for example, the interaction is perfectly elastic or regarded as point-like collisions.
Solid-state physics is the study of rigid matter, or solids, through methods such as solid-state chemistry, quantum mechanics, crystallography, electromagnetism, and metallurgy. It is the largest branch of condensed matter physics. Solid-state physics studies how the large-scale properties of solid materials result from their atomic-scale properties. Thus, solid-state physics forms a theoretical basis of materials science. Along with solid-state chemistry, it also has direct applications in the technology of transistors and semiconductors.

The Fermi level of a solid-state body is the thermodynamic work required to add one electron to the body. It is a thermodynamic quantity usually denoted by µ or EF for brevity. The Fermi level does not include the work required to remove the electron from wherever it came from. A precise understanding of the Fermi level—how it relates to electronic band structure in determining electronic properties; how it relates to the voltage and flow of charge in an electronic circuit—is essential to an understanding of solid-state physics.

A Fermi gas is an idealized model, an ensemble of many non-interacting fermions. Fermions are particles that obey Fermi–Dirac statistics, like electrons, protons, and neutrons, and, in general, particles with half-integer spin. These statistics determine the energy distribution of fermions in a Fermi gas in thermal equilibrium, and is characterized by their number density, temperature, and the set of available energy states. The model is named after the Italian physicist Enrico Fermi.

Fermi liquid theory is a theoretical model of interacting fermions that describes the normal state of the conduction electrons in most metals at sufficiently low temperatures. The theory describes the behavior of many-body systems of particles in which the interactions between particles may be strong. The phenomenological theory of Fermi liquids was introduced by the Soviet physicist Lev Davidovich Landau in 1956, and later developed by Alexei Abrikosov and Isaak Khalatnikov using diagrammatic perturbation theory. The theory explains why some of the properties of an interacting fermion system are very similar to those of the ideal Fermi gas, and why other properties differ.
In electromagnetism, the magnetic susceptibility is a measure of how much a material will become magnetized in an applied magnetic field. It is the ratio of magnetization M to the applied magnetizing field intensity H. This allows a simple classification, into two categories, of most materials' responses to an applied magnetic field: an alignment with the magnetic field, χ > 0, called paramagnetism, or an alignment against the field, χ < 0, called diamagnetism.

In electromagnetism, permeability is the measure of magnetization produced in a material in response to an applied magnetic field. Permeability is typically represented by the (italicized) Greek letter μ. It is the ratio of the magnetic induction to the magnetizing field as a function of the field in a material. The term was coined by William Thomson, 1st Baron Kelvin in 1872, and used alongside permittivity by Oliver Heaviside in 1885. The reciprocal of permeability is magnetic reluctivity.
In solid-state physics, the free electron model is a quantum mechanical model for the behaviour of charge carriers in a metallic solid. It was developed in 1927, principally by Arnold Sommerfeld, who combined the classical Drude model with quantum mechanical Fermi–Dirac statistics and hence it is also known as the Drude–Sommerfeld model.
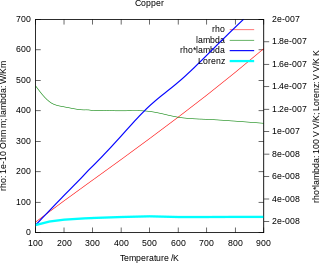
In physics, the Wiedemann–Franz law states that the ratio of the electronic contribution of the thermal conductivity (κ) to the electrical conductivity (σ) of a metal is proportional to the temperature (T).

Electron paramagnetic resonance (EPR) or electron spin resonance (ESR) spectroscopy is a method for studying materials that have unpaired electrons. The basic concepts of EPR are analogous to those of nuclear magnetic resonance (NMR), but the spins excited are those of the electrons instead of the atomic nuclei. EPR spectroscopy is particularly useful for studying metal complexes and organic radicals. EPR was first observed in Kazan State University by Soviet physicist Yevgeny Zavoisky in 1944, and was developed independently at the same time by Brebis Bleaney at the University of Oxford.
The Kadowaki–Woods ratio is the ratio of A, the quadratic term of the resistivity and γ2, the linear term of the specific heat. This ratio is found to be a constant for transition metals, and for heavy-fermion compounds, although at different values.
The De Haas–Van Alphen effect, often abbreviated to DHVA, is a quantum mechanical effect in which the magnetic susceptibility of a pure metal crystal oscillates as the intensity of the magnetic field B is increased. It can be used to determine the Fermi surface of a material. Other quantities also oscillate, such as the electrical resistivity, specific heat, and sound attenuation and speed. It is named after Wander Johannes de Haas and his student Pieter M. van Alphen. The DHVA effect comes from the orbital motion of itinerant electrons in the material. An equivalent phenomenon at low magnetic fields is known as Landau diamagnetism.
In Materials Science, heavy fermion materials are a specific type of intermetallic compound, containing elements with 4f or 5f electrons in unfilled electron bands. Electrons are one type of fermion, and when they are found in such materials, they are sometimes referred to as heavy electrons. Heavy fermion materials have a low-temperature specific heat whose linear term is up to 1000 times larger than the value expected from the free electron model. The properties of the heavy fermion compounds often derive from the partly filled f-orbitals of rare-earth or actinide ions, which behave like localized magnetic moments.
Magnetochemistry is concerned with the magnetic properties of chemical compounds. Magnetic properties arise from the spin and orbital angular momentum of the electrons contained in a compound. Compounds are diamagnetic when they contain no unpaired electrons. Molecular compounds that contain one or more unpaired electrons are paramagnetic. The magnitude of the paramagnetism is expressed as an effective magnetic moment, μeff. For first-row transition metals the magnitude of μeff is, to a first approximation, a simple function of the number of unpaired electrons, the spin-only formula. In general, spin–orbit coupling causes μeff to deviate from the spin-only formula. For the heavier transition metals, lanthanides and actinides, spin–orbit coupling cannot be ignored. Exchange interaction can occur in clusters and infinite lattices, resulting in ferromagnetism, antiferromagnetism or ferrimagnetism depending on the relative orientations of the individual spins.
In condensed matter physics, a quantum spin liquid is a phase of matter that can be formed by interacting quantum spins in certain magnetic materials. Quantum spin liquids (QSL) are generally characterized by their long-range quantum entanglement, fractionalized excitations, and absence of ordinary magnetic order.

In condensed matter physics, quantum oscillations describes a series of related experimental techniques used to map the Fermi surface of a metal in the presence of a strong magnetic field. These techniques are based on the principle of Landau quantization of Fermions moving in a magnetic field. For a gas of free fermions in a strong magnetic field, the energy levels are quantized into bands, called the Landau levels, whose separation is proportional to the strength of the magnetic field. In a quantum oscillation experiment, the external magnetic field is varied, which causes the Landau levels to pass over the Fermi surface, which in turn results in oscillations of the electronic density of states at the Fermi level; this produces oscillations in the many material properties which depend on this, including resistance, Hall resistance, and magnetic susceptibility. Observation of quantum oscillations in a material is considered a signature of Fermi liquid behaviour.
In condensed matter and atomic physics, Van Vleck paramagnetism refers to a positive and temperature-independent contribution to the magnetic susceptibility of a material, derived from second order corrections to the Zeeman interaction. The quantum mechanical theory was developed by John Hasbrouck Van Vleck between the 1920s and the 1930s to explain the magnetic response of gaseous nitric oxide and of rare-earth salts. Alongside other magnetic effects like Paul Langevin's formulas for paramagnetism and diamagnetism, Van Vleck discovered an additional paramagnetic contribution of the same order as Langevin's diamagnetism. Van Vleck contribution is usually important for systems with one electron short of being half filled and this contribution vanishes for elements with closed shells.









