Structure and properties
All-trans-lycopene with canonical numbering:


| Structure and properties | |
|---|---|
| Index of refraction, nD | ? |
| Dielectric constant, εr | ? ε0 at ? °C |
| Bond strength | ? |
| Bond length | ? |
| Bond angle | ? |
| Magnetic susceptibility | ? |
This page provides supplementary chemical data on lycopene.
The handling of this chemical may incur notable safety precautions. It is highly recommend that you seek the Material Safety Datasheet (MSDS) for this chemical from a reliable source and follow its directions.
All-trans-lycopene with canonical numbering:


| Structure and properties | |
|---|---|
| Index of refraction, nD | ? |
| Dielectric constant, εr | ? ε0 at ? °C |
| Bond strength | ? |
| Bond length | ? |
| Bond angle | ? |
| Magnetic susceptibility | ? |
To date, no X-ray crystal structure of lycopene has been reported.

| UV-Vis | |
|---|---|
| λmax | 443, 471, 502 nm in hexane |
| Extinction coefficient, ε | 1.72 × 105 L•mol−1•cm−1(at 502 nm) [3] |
| IR | |
| Major absorption bands | ? cm −1 |
| NMR | |
| Proton NMR | |
| Carbon-13 NMR | |
| Other NMR data | |
| MS | |
| Masses of main fragments | |

Lycopene is an organic compound classified as a tetraterpene and a carotene. Lycopene is a bright red carotenoid hydrocarbon found in tomatoes and other red fruits and vegetables.
Neurochemistry is the study of chemicals, including neurotransmitters and other molecules such as psychopharmaceuticals and neuropeptides, that control and influence the physiology of the nervous system. This particular field within neuroscience examines how neurochemicals influence the operation of neurons, synapses, and neural networks. Neurochemists analyze the biochemistry and molecular biology of organic compounds in the nervous system, and their roles in such neural processes including cortical plasticity, neurogenesis, and neural differentiation.

Dicyclopentadiene, abbreviated DCPD, is a chemical compound with formula C10H12. At room temperature, it is a white brittle wax, although lower purity samples can be straw coloured liquids. The pure material smells somewhat of soy wax or camphor, with less pure samples possessing a stronger acrid odor. Its energy density is 10,975 Wh/l. Dicyclopentadiene is a co-produced in large quantities in the steam cracking of naphtha and gas oils to ethylene. The major use is in resins, particularly, unsaturated polyester resins. It is also used in inks, adhesives, and paints.
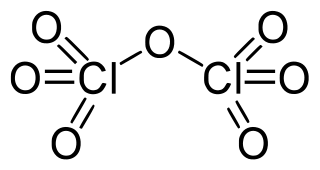
Dichlorine heptoxide is the chemical compound with the formula Cl2O7. This chlorine oxide is the anhydride of perchloric acid. It is produced by the careful distillation of perchloric acid in the presence of the dehydrating agent phosphorus pentoxide:

In chemistry, a phosphaalkyne is an organophosphorus compound containing a triple bond between phosphorus and carbon with the general formula R-C≡P. Phosphaalkynes are the heavier congeners of nitriles, though, due to the similar electronegativities of phosphorus and carbon, possess reactivity patterns reminiscent of alkynes. Due to their high reactivity, phosphaalkynes are not found naturally on earth, but the simplest phosphaalkyne, phosphaethyne (H-C≡P) has been observed in the interstellar medium.
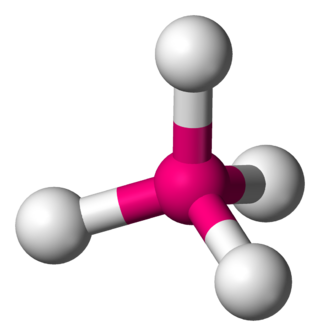
In a tetrahedral molecular geometry, a central atom is located at the center with four substituents that are located at the corners of a tetrahedron. The bond angles are cos−1(−1⁄3) = 109.4712206...° ≈ 109.5° when all four substituents are the same, as in methane as well as its heavier analogues. Methane and other perfectly symmetrical tetrahedral molecules belong to point group Td, but most tetrahedral molecules have lower symmetry. Tetrahedral molecules can be chiral.

Germabenzene (C5H6Ge) is the parent representative of a group of chemical compounds containing in their molecular structure a benzene ring with a carbon atom replaced by a germanium atom. Germabenzene itself has been studied theoretically, and synthesized with a bulky 2,4,6-tris[bis(trimethylsilyl)methyl]phenyl or Tbt group. Also, stable naphthalene derivatives do exist in the laboratory such as the 2-germanaphthalene-containing substance represented below. The germanium to carbon bond in this compound is shielded from potential reactants by a Tbt group. This compound is aromatic just as the other carbon group representatives silabenzene and stannabenzene.

Dioxane tetraketone (or 1,4-dioxane-2,3,5,6-tetrone) is an organic compound with the formula C4O6. It is an oxide of carbon (an oxocarbon), which can be viewed as the fourfold ketone of dioxane. It can also be viewed as the cyclic dimer of oxiranedione (C2O3), the hypothetical anhydride of oxalic acid.

Benzotriyne or cyclo[6]carbon is a hypothetical compound, an allotrope of carbon with molecular formula C6. The molecule is a ring of six carbon atoms, connected by alternating triple and single bonds. It is, therefore, a potential member of the cyclo[n]carbon family.
Angela K. Wilson is an American scientist and former (2022) President of the American Chemical Society. She currently serves as the John A. Hannah Distinguished Professor of Chemistry, Associate Dean for Strategic Initiatives in the College of Natural Sciences, and Director of the MSU Center for Quantum Computing, Science, and Engineering (MSU-Q) at Michigan State University.

Oxirene is a heterocyclic chemical compound which contains an unsaturated three-membered ring containing two carbon atoms and one oxygen atom. The molecule was synthesized in low temperature ices and detected upon sublimation by isomer selective photoionization reflectron time-of-flight mass spectrometry.

Bicyclopropenyl (bicycloprop-2-enyl, C6H6) is an organic compound and one of several valence isomers of benzene. The molecule can be described as two coupled cyclopropene units. The positions of the alkene groups can vary and therefore two other isomers are known: bicycloprop-1,2-enyl and bicyclopropen-1-yl.
The ONIOM method is a computational approach developed by Morokuma and co-workers. ONIOM is a hybrid method that enables different ab initio, semi-empirical, or molecular mechanics methods to be applied to different parts of a molecule/system in combination to produce reliable geometry and energy at reduced computational cost.
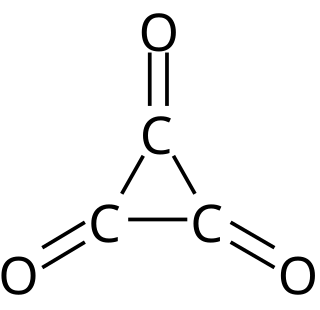
Cyclopropanetrione or trioxocyclopropane is a little-known oxide of carbon with formula C3O3. It consists of a ring of three carbon atoms each attached to an oxygen atom with a double bond. Alternately, it can be thought as a trimer of carbon monoxide. This compound is thermodynamically unstable and has not been produced in bulk. However, it has been detected using mass spectrometry.
In theoretical chemistry, the charge-shift bond is a proposed new class of chemical bonds that sits alongside the three familiar families of covalent, ionic, and metallic bonds where electrons are shared or transferred respectively. The charge shift bond derives its stability from the resonance of ionic forms rather than the covalent sharing of electrons which are often depicted as having electron density between the bonded atoms. A feature of the charge shift bond is that the predicted electron density between the bonded atoms is low. It has long been known from experiment that the accumulation of electric charge between the bonded atoms is not necessarily a feature of covalent bonds.

Dinitrogen dioxide is an inorganic compound having molecular formula N
2O
2. Many structural isomers are possible. The covalent bonding pattern O=N–N=O is predicted to be the most stable isomer based on ab initio calculations and is the only one that has been experimentally produced. In the solid form, the molecules have C2v symmetry: the entire structure is planar, with the two oxygen atoms cis across the N–N bond. The O–N distance is 1.15 Å, the N–N distance is 2.33 Å, and the O=N–N angle is 95°.

1,3-Difluoro-trisulfane-1,1-difluoride is an inorganic molecular substance with the structure SF3SSF, consisting of sulfur in a low oxidation state with fluorine. The compound consists of a chain of three sulfur atoms, with three fluorine atoms bonded to the sulfur on one end and the fourth fluorine bonded to the sulfur on the other end. It has a melting point of -62 °C and a boiling point of 94 °C. As a gas, it is unstable and breaks up to form SSF2 and SF4.
Péter R. Surján is a Hungarian theoretical chemist who is known for his research on application of the theory of second quantization in quantum chemistry. In 2016 a festschrift from Theoretical Chemistry Accounts journal was published in his name which is also published as a book in Highlights in Theoretical Chemistry series by the Springer Nature. He is currently a professor and a former dean of the Faculty of Science of the Eötvös Loránd University.
Azidotetrazolate (CN7−) is an anion which forms a highly explosive series of salts. The ion is made by removing a proton from 5-azido-1H-tetrazole. The molecular structure contains a five-membered ring with four nitrogen atoms, and an azido side chain connected to the carbon atom. Several salts exist, but they are unstable and spontaneously explode. Rubidium azidotetrazolate was so unstable that it explodes while crystallizing. The potassium and caesium salt also spontaneously explode when dry.
Pentanenitrile, valeronitrile or butyl cyanide is a nitrile with the formula C4H9CN. This can be written BuCN, with Bu representing an n-butyl (linear butyl group).