
The alkali metals consist of the chemical elements lithium (Li), sodium (Na), potassium (K), rubidium (Rb), caesium (Cs), and francium (Fr). Together with hydrogen they constitute group 1, which lies in the s-block of the periodic table. All alkali metals have their outermost electron in an s-orbital: this shared electron configuration results in their having very similar characteristic properties. Indeed, the alkali metals provide the best example of group trends in properties in the periodic table, with elements exhibiting well-characterised homologous behaviour. This family of elements is also known as the lithium family after its leading element.

Sodium hydroxide, also known as lye and caustic soda, is an inorganic compound with the formula NaOH. It is a white solid ionic compound consisting of sodium cations Na+ and hydroxide anions OH−.

Sodium carbonate is the inorganic compound with the formula Na2CO3 and its various hydrates. All forms are white, odourless, water-soluble salts that yield alkaline solutions in water. Historically, it was extracted from the ashes of plants grown in sodium-rich soils, and because the ashes of these sodium-rich plants were noticeably different from ashes of wood, sodium carbonate became known as "soda ash". It is produced in large quantities from sodium chloride and limestone by the Solvay process, as well as by carbonating sodium hydroxide which is made using the chloralkali process.
A substance is pyrophoric if it ignites spontaneously in air at or below 54 °C (129 °F) or within 5 minutes after coming into contact with air. Examples are organolithium compounds and triethylborane. Pyrophoric materials are often water-reactive as well and will ignite when they contact water or humid air. They can be handled safely in atmospheres of argon or nitrogen. Class D fire extinguishers are designated for use in fires involving pyrophoric materials. A related concept is hypergolicity, in which two compounds spontaneously ignite when mixed.

Silica gel is an amorphous and porous form of silicon dioxide (silica), consisting of an irregular tridimensional framework of alternating silicon and oxygen atoms with nanometer-scale voids and pores. The voids may contain water or some other liquids, or may be filled by gas or vacuum. In the last case, the material is properly called silica xerogel.
Sodium silicate is a generic name for chemical compounds with the formula Na
2xSi
yO
2y+x or (Na
2O)
x·(SiO
2)
y, such as sodium metasilicate, sodium orthosilicate, and sodium pyrosilicate. The anions are often polymeric. These compounds are generally colorless transparent solids or white powders, and soluble in water in various amounts.

A molecular sieve is a material with pores, having uniform size comparable to that of individual molecules, linking the interior of the solid to its exterior. These materials embody the molecular sieve effect, the preferential sieving of molecules larger than the pores. Many kinds of materials exhibit some molecular sieves, but zeolites dominate the field. Zeolites are almost always aluminosilicates, or variants where some or all of the Si or Al centers are replaced by similarly charged elements.

In organic chemistry, a radical anion is a free radical species that carries a negative charge. Radical anions are encountered in organic chemistry as reduced derivatives of polycyclic aromatic compounds, e.g. sodium naphthenide. An example of a non-carbon radical anion is the superoxide anion, formed by transfer of one electron to an oxygen molecule. Radical anions are typically indicated by .

In the area of solid state chemistry, graphite intercalation compounds are a family of materials prepared from graphite. In particular, the sheets of carbon that comprise graphite can be pried apart by the insertion (intercalation) of ions. The graphite is viewed as a host and the inserted ions as guests. The materials have the formula (guest)Cn where n ≥ 6. The insertion of the guests increases the distance between the carbon sheets. Common guests are reducing agents such as alkali metals. Strong oxidants also intercalate into graphite. Intercalation involves electron transfer into or out of the carbon sheets. So, in some sense, graphite intercalation compounds are salts. Intercalation is often reversible: the inserted ions can be removed and the sheets of carbon collapse to a graphite-like structure.

Hexafluorosilicic acid is an inorganic compound with the chemical formula H
2SiF
6. Aqueous solutions of hexafluorosilicic acid consist of salts of the cation and hexafluorosilicate anion. These salts and their aqueous solutions are colorless.

Sodium formate, HCOONa, is the sodium salt of formic acid, HCOOH. It usually appears as a white deliquescent powder.
Sodium atoms have 11 electrons, one more than the stable configuration of the noble gas neon. As a result, sodium usually forms ionic compounds involving the Na+ cation. Sodium is a reactive alkali metal and is much more stable in ionic compounds. It can also form intermetallic compounds and organosodium compounds. Sodium compounds are often soluble in water.
A pyrotechnic composition is a substance or mixture of substances designed to produce an effect by heat, light, sound, gas/smoke or a combination of these, as a result of non-detonative self-sustaining exothermic chemical reactions. Pyrotechnic substances do not rely on oxygen from external sources to sustain the reaction.

A Rieke metal is a highly reactive metal powder generated by reduction of a metal salt with an alkali metal. These materials are named after Reuben D. Rieke, who first described along with an associate in 1972 the recipes for their preparation. In 1974 he told about Rieke-magnesium. A 1989 paper by Rieke lists several metals that are allowed by the periodic table to be produced by his process: Cd, Zn, Ni, Pt, Pd, Fe, In, Tl, Co, Cr, Mo, W, Cu, which in turn are called Rieke-nickel, Rieke-platinum, etc.
A geopolymer is a vague pseudo-chemical term used to describe inorganic, typically bulk ceramic-like material that forms covalently bonded, non-crystalline (amorphous) networks, often intermingled with other phases. Many geopolymers may also be classified as alkali-activated cements or acid-activated binders. They are mainly produced by a chemical reaction between a chemically reactive aluminosilicate powder e.g. metakaolin or other clay-derived powders, natural pozzolan, or suitable glasses, and an aqueous solution that causes this powder to react and re-form into a solid monolith. The most common pathway to produce geopolymers is by the reaction of metakaolin with sodium silicate, which is an alkaline solution, but other processes are also possible.

The oxidation state of oxygen is −2 in almost all known compounds of oxygen. The oxidation state −1 is found in a few compounds such as peroxides. Compounds containing oxygen in other oxidation states are very uncommon: −1⁄2 (superoxides), −1⁄3 (ozonides), 0, +1⁄2 (dioxygenyl), +1, and +2.
Porous glass is glass that includes pores, usually in the nanometre- or micrometre-range, commonly prepared by one of the following processes: through metastable phase separation in borosilicate glasses (such as in their system SiO2-B2O3-Na2O), followed by liquid extraction of one of the formed phases; through the sol-gel process; or simply by sintering glass powder.

Ammonium thiocyanate is an inorganic compound with the formula [NH4]+[SCN]−. It is an ammonium salt of thiocyanic acid. It consists of ammonium cations [NH4]+ and thiocyanate anions [SCN]−.

Compounds of lead exist with lead in two main oxidation states: +2 and +4. The former is more common. Inorganic lead(IV) compounds are typically strong oxidants or exist only in highly acidic solutions.
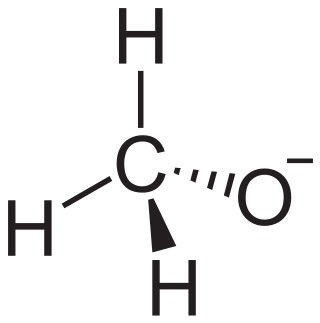
In chemistry, an alkoxide is the conjugate base of an alcohol and therefore consists of an organic group bonded to a negatively charged oxygen atom. They are written as RO−, where R is the organyl substituent. Alkoxides are strong bases and, when R is not bulky, good nucleophiles and good ligands. Alkoxides, although generally not stable in protic solvents such as water, occur widely as intermediates in various reactions, including the Williamson ether synthesis. Transition metal alkoxides are widely used for coatings and as catalysts.














