Related Research Articles

Beryllium is a chemical element with the symbol Be and atomic number 4. It is a steel-gray, strong, lightweight and brittle alkaline earth metal. It is a divalent element that occurs naturally only in combination with other elements to form minerals. Notable gemstones high in beryllium include beryl and chrysoberyl. It is a relatively rare element in the universe, usually occurring as a product of the spallation of larger atomic nuclei that have collided with cosmic rays. Within the cores of stars, beryllium is depleted as it is fused into heavier elements. Beryllium constitutes about 0.0004 percent by mass of Earth's crust. The world's annual beryllium production of 220 tons is usually manufactured by extraction from the mineral beryl, a difficult process because beryllium bonds strongly to oxygen.

Hafnium is a chemical element with the symbol Hf and atomic number 72. A lustrous, silvery gray, tetravalent transition metal, hafnium chemically resembles zirconium and is found in many zirconium minerals. Its existence was predicted by Dmitri Mendeleev in 1869, though it was not identified until 1923, by Dirk Coster and George de Hevesy, making it the second-last stable element to be discovered. Hafnium is named after Hafnia, the Latin name for Copenhagen, where it was discovered.

Magnesium is a chemical element with the symbol Mg and atomic number 12. It is a shiny gray solid which bears a close physical resemblance to the other five elements in the second column of the periodic table: all group 2 elements have the same electron configuration in the outer electron shell and a similar crystal structure.

Zirconium is a chemical element with the symbol Zr and atomic number 40. The name zirconium is taken from the name of the mineral zircon (the word is related to Persian zargun, the most important source of zirconium. It is a lustrous, grey-white, strong transition metal that closely resembles hafnium and, to a lesser extent, titanium. Zirconium is mainly used as a refractory and opacifier, although small amounts are used as an alloying agent for its strong resistance to corrosion. Zirconium forms a variety of inorganic and organometallic compounds such as zirconium dioxide and zirconocene dichloride, respectively. Five isotopes occur naturally, four of which are stable. Zirconium compounds have no known biological role.

Zirconium dioxide, sometimes known as zirconia, is a white crystalline oxide of zirconium. Its most naturally occurring form, with a monoclinic crystalline structure, is the mineral baddeleyite. A dopant stabilized cubic structured zirconia, cubic zirconia, is synthesized in various colours for use as a gemstone and a diamond simulant.
The periodic table is laid out in rows to illustrate recurring (periodic) trends in the chemical behaviour of the elements as their atomic number increases: a new row is begun when chemical behaviour begins to repeat, meaning that elements with similar behaviour fall into the same vertical columns. The fifth period contains 18 elements, beginning with rubidium and ending with xenon. As a rule, period 5 elements fill their 5s shells first, then their 4d, and 5p shells, in that order; however, there are exceptions, such as rhodium.
A substance is pyrophoric if it ignites spontaneously in air at or below 54 °C (129 °F) or within 5 minutes after coming into contact with air. Examples are iron sulfide (FeS) and many reactive metals including plutonium and uranium, when powdered or thinly sliced. Pyrophoric materials are often water-reactive as well and will ignite when they contact water or humid air. They can be handled safely in atmospheres of argon or nitrogen. Class D fire extinguishers are designated for use in fires involving pyrophoric materials.

Magnox is a type of nuclear power/production reactor that was designed to run on natural uranium with graphite as the moderator and carbon dioxide gas as the heat exchange coolant. It belongs to the wider class of gas-cooled reactors. The name comes from the magnesium-aluminium alloy used to clad the fuel rods inside the reactor. Like most other "Generation I nuclear reactors", the Magnox was designed with the dual purpose of producing electrical power and plutonium-239 for the nascent nuclear weapons program in Britain. The name refers specifically to the United Kingdom design but is sometimes used generically to refer to any similar reactor.

Group 4 is the second group of transition metals in the periodic table. It contains the four elements titanium (Ti), zirconium (Zr), hafnium (Hf), and rutherfordium (Rf). The group is also called the titanium group or titanium family after its lightest member.
Ames Laboratory is a United States Department of Energy national laboratory located in Ames, Iowa and affiliated with Iowa State University. It is a top level national laboratory for new research in various domains concerning national security and resource management. The laboratory conducts research into various areas of national concern, including the synthesis and study of new materials, energy resources, high-speed computer design, and environmental cleanup and restoration. It is located on the campus of Iowa State University.
Elektron is the registered trademark of a wide range of magnesium alloys manufactured by a British company Magnesium Elektron Limited.
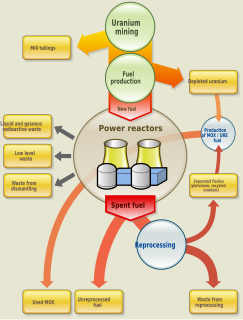
Nuclear fuel is material used in nuclear power stations to produce heat to power turbines. Heat is created when nuclear fuel undergoes nuclear fission.
Zirconium alloys are solid solutions of zirconium or other metals, a common subgroup having the trade mark Zircaloy. Zirconium has very low absorption cross-section of thermal neutrons, high hardness, ductility and corrosion resistance. One of the main uses of zirconium alloys is in nuclear technology, as cladding of fuel rods in nuclear reactors, especially water reactors. A typical composition of nuclear-grade zirconium alloys is more than 95 weight percent zirconium and less than 2% of tin, niobium, iron, chromium, nickel and other metals, which are added to improve mechanical properties and corrosion resistance.
The Distillers Company Limited was a leading Scottish drinks and pharmaceutical company and, at one time, a constituent of the FTSE 100 Index. It was taken over by Guinness & Co. in 1986 in a transaction which was later found to be involved in fraudulent activity, becoming known as the Guinness share-trading fraud.
Magnesium alloys are mixtures of magnesium with other metals, often aluminium, zinc, manganese, silicon, copper, rare earths and zirconium. Magnesium alloys have a hexagonal lattice structure, which affects the fundamental properties of these alloys. Plastic deformation of the hexagonal lattice is more complicated than in cubic latticed metals like aluminium, copper and steel; therefore, magnesium alloys are typically used as cast alloys, but research of wrought alloys has been more extensive since 2003. Cast magnesium alloys are used for many components of modern automobiles and have been used in some high-performance vehicles; die-cast magnesium is also used for camera bodies and components in lenses.

The UNGG is an obsolete nuclear power reactor design developed in France. It was graphite moderated, cooled by carbon dioxide, and fueled with natural uranium metal. The first generation of French nuclear power stations were UNGGs, as was Vandellos unit 1 in Spain. Of ten units built, all were shut down by end 1994, most for economic reasons due to staffing costs.

MAHLE GmbH is a German automotive parts manufacturer based in Stuttgart, Germany. It is one of the largest automotive suppliers worldwide. As a manufacturer of components and systems for the combustion engine and its periphery, the company is one of the three largest systems suppliers worldwide for engine systems, filtration, electrics, mechatronics, and thermal management. In 2018, Mahle GmbH sales amounted to over €12.5 billion.

Hunterston A nuclear power station is a decommissioned Magnox nuclear power station located at Hunterston in Ayrshire, Scotland, adjacent to Hunterston B. The ongoing decommissioning process is being managed by Nuclear Decommissioning Authority (NDA) subsidiary Magnox Ltd.

Luch Scientific Production Association or NPO Luch is a company based in Podolsk, Russia. It is part of Rosatom.
References
- 1 2 "MEL Chemicals Company History". Archived from the original on 2012-10-16. Retrieved 2013-01-25.
- ↑ Ball, C J P (February 1957). "The History of Magnesium". Journal of the American Society for Naval Engineers. 69 (1): 81–94. doi:10.1111/j.1559-3584.1957.tb04052.x.
- ↑ "Memoirs of a Wartime Welder, part 1 of 4". WW2 People's War. BBC. 24 December 2005. Retrieved 12 May 2021.
- ↑ Barnes, Russell W. "The WWII plant at Harrington for extracting magnesium from seawater" . Retrieved 12 May 2021.
- ↑ "Distillers Co". Grace's Guide. Retrieved 12 May 2021.
- ↑ General Nuclear Engineering Corporation (1957). Power Reactor Technology. Dunedin, Florida: United States Atomic Energy Commission. p. 32.
- ↑ "Magnesium Alloys - Characteristics and Uses". Nuclear Power. Retrieved 12 May 2021.
- ↑ "About Us | Metal & Chemical Manufacturer". Luxfer MEL Technologies. Retrieved 12 May 2021.