
Collagen is the main structural protein in the extracellular matrix found in the body's various connective tissues. As the main component of connective tissue, it is the most abundant protein in mammals, making up from 25% to 35% of the whole-body protein content. Collagen consists of amino acids bound together to form a triple helix of elongated fibril known as a collagen helix. It is mostly found in connective tissue such as cartilage, bones, tendons, ligaments, and skin. Vitamin C is vital for collagen synthesis, and Vitamin E improves the production of collagen.

Histology, also known as microscopic anatomy or microanatomy, is the branch of biology that studies the microscopic anatomy of biological tissues. Histology is the microscopic counterpart to gross anatomy, which looks at larger structures visible without a microscope. Although one may divide microscopic anatomy into organology, the study of organs, histology, the study of tissues, and cytology, the study of cells, modern usage places all of these topics under the field of histology. In medicine, histopathology is the branch of histology that includes the microscopic identification and study of diseased tissue. In the field of paleontology, the term paleohistology refers to the histology of fossil organisms.

Staining is a technique used to enhance contrast in samples, generally at the microscopic level. Stains and dyes are frequently used in histology, in cytology, and in the medical fields of histopathology, hematology, and cytopathology that focus on the study and diagnoses of diseases at the microscopic level. Stains may be used to define biological tissues, cell populations, or organelles within individual cells.

Histopathology is the microscopic examination of tissue in order to study the manifestations of disease. Specifically, in clinical medicine, histopathology refers to the examination of a biopsy or surgical specimen by a pathologist, after the specimen has been processed and histological sections have been placed onto glass slides. In contrast, cytopathology examines free cells or tissue micro-fragments.
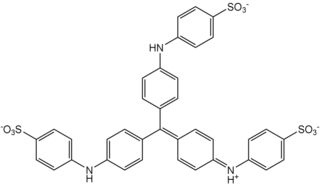
Methyl blue is a chemical compound with the molecular formula C37H27N3Na2O9S3. It is used as a stain in histology, and stains collagen blue in tissue sections. It can be used in some differential staining techniques such as Mallory's connective tissue stain and Gömöri trichrome stain, and can be used to mediate electron transfer in microbial fuel cells. Fungal cell walls are also stained by methyl blue.
Trichrome staining is a histological staining method that uses two or more acid dyes in conjunction with a polyacid. Staining differentiates tissues by tinting them in contrasting colours. It increases the contrast of microscopic features in cells and tissues, which makes them easier to see when viewed through a microscope.

Charles Philippe Leblond was a pioneer of cell biology and stem cell research and a Canadian former professor of anatomy. Leblond is notable for developing autoradiography and his work showing how cells continuously renew themselves, regardless of age.

Reticular fibers, reticular fibres or reticulin is a type of fiber in connective tissue composed of type III collagen secreted by reticular cells. They are mainly composed of reticulin protein and form a network or mesh. Reticular fibers crosslink to form a fine meshwork (reticulin). This network acts as a supporting mesh in soft tissues such as liver, bone marrow, and the tissues and organs of the lymphatic system.

Masson's trichrome is a three-colour staining procedure used in histology. The recipes evolved from Claude L. Pierre Masson's (1880–1959) original formulation have different specific applications, but all are suited for distinguishing cells from surrounding connective tissue.

Aniline Blue WS, also called aniline blue, diphenylamine blue, China blue, or Soluble blue, is a mixture of methyl blue and water blue. It may also be either one of them. It is a soluble dye used as a biological dye, in fluorescence microscopy, appearing a yellow-green colour after excitation with violet light. It is a mixture of the trisulfonates of triphenyl rosaniline and of diphenyl rosaniline.

Water blue, also known as aniline blue, Acid blue 22, Soluble Blue 3M, Marine Blue V, or C.I. 42755, is a chemical compound used as a stain in histology. Water blue stains collagen blue in tissue sections. It is soluble in water and slightly soluble in ethanol.

Hematoxylin and eosin stain is one of the principal tissue stains used in histology. It is the most widely used stain in medical diagnosis and is often the gold standard. For example, when a pathologist looks at a biopsy of a suspected cancer, the histological section is likely to be stained with H&E.

Phosphotungstic acid (PTA) or tungstophosphoric acid (TPA), is a heteropoly acid with the chemical formula H3PW12O40]. It forms hydrates H3[PW12O40]·nH2O. It is normally isolated as the n = 24 hydrate but can be desiccated to the hexahydrate (n = 6). EPTA is the name of ethanolic phosphotungstic acid, its alcohol solution used in biology. It has the appearance of small, colorless-grayish or slightly yellow-green crystals, with melting point 89 °C (24 H2O hydrate). It is odorless and soluble in water (200 g/100 ml). It is not especially toxic, but is a mild acidic irritant. The compound is known by a variety of names and acronyms (see 'other names' section of infobox).
In the fields of histology, pathology, and cell biology, fixation is the preservation of biological tissues from decay due to autolysis or putrefaction. It terminates any ongoing biochemical reactions and may also increase the treated tissues' mechanical strength or stability. Tissue fixation is a critical step in the preparation of histological sections, its broad objective being to preserve cells and tissue components and to do this in such a way as to allow for the preparation of thin, stained sections. This allows the investigation of the tissues' structure, which is determined by the shapes and sizes of such macromolecules as proteins and nucleic acids.
Van Gieson's stain is a mixture of picric acid and acid fuchsin. It is the simplest method of differential staining of collagen and other connective tissue. It was introduced to histology by American neuropsychiatrist and pathologist Ira Van Gieson.

Acid fuchsin or fuchsine acid, (also called Acid Violet 19 and C.I. 42685) is an acidic magenta dye with the chemical formula C20H17N3Na2O9S3. It is a sodium sulfonate derivative of fuchsine. Acid fuchsin has wide use in histology, and is one of the dyes used in Masson's trichrome stain. This method is commonly used to stain cytoplasm and nuclei of tissue sections in the histology laboratory in order to distinguish muscle from collagen. The muscle stains red with the acid fuchsin, and the collagen is stained green or blue with Light Green SF yellowish or methyl blue. It can also be used to identify growing bacteria.
Lillie's trichrome is a combination of dyes used in histology.
Trichrome stains are staining methods in which three anionic dyes are used, in conjunction with either phosphomolybdic acid (PMA), phosphotungstic acid (PTA), or a mixture of these heteropolyacids. Probably the first trichrome method was that of Frank B Mallory, an American pathologist, first published in 1900. Unfortunately, none of Mallory's publications provide any explanation of the rationales of either his trichrome or his phosphotungstic acid-haematoxylin (PTAH) method. Nobody knows why Mallory introduced heteropolyacids into microtechnique.
Bouin solution, or Bouin's solution, is a compound fixative used in histology. It was invented by French biologist Pol Bouin and is composed of picric acid, acetic acid and formaldehyde in an aqueous solution. Bouin's fluid is especially useful for fixation of gastrointestinal tract biopsies because this fixative allows crisper and better nuclear staining than 10% neutral-buffered formalin. It is not a good fixative when tissue ultrastructure must be preserved for electron microscopy. However, it is a good fixative when tissue structure with a soft and delicate texture must be preserved. The acetic acid in this fixative lyses red blood cells and dissolves small iron and calcium deposits in tissue. A variant in which the acetic acid is replaced with formic acid can be used for both fixation of tissue and decalcification. The effects of the three chemicals in Bouin solution balance each other. Formalin causes cytoplasm to become basophilic but this effect is balanced by the effect of the picric acid. This results in excellent nuclear and cytoplasmic H&E staining. The tissue hardening effect of formalin is balanced by the soft tissue fixation of picric and acetic acids. The tissue swelling effect of acetic acid is balanced by the tissue shrinking effect of picric acid.
Verhoeff's stain, also known as Verhoeff's elastic stain (VEG) or Verhoeff–Van Gieson stain (VVG), is a staining protocol used in histology, developed by American ophthalmic surgeon and pathologist Frederick Herman Verhoeff (1874–1968) in 1908. The formulation is used to demonstrate normal or pathologic elastic fibers.














