Eosin is the name of several fluorescent acidic compounds which bind to and form salts with basic, or eosinophilic, compounds like proteins containing amino acid residues such as arginine and lysine, and stains them dark red or pink as a result of the actions of bromine on eosin. In addition to staining proteins in the cytoplasm, it can be used to stain collagen and muscle fibers for examination under the microscope. Structures, that stain readily with eosin, are termed eosinophilic. In the field of histology, Eosin Y is the form of eosin used most often as a histologic stain.

Methyl violet is a family of organic compounds that are mainly used as dyes. Depending on the number of attached methyl groups, the color of the dye can be altered. Its main use is as a purple dye for textiles and to give deep violet colors in paint and ink, it is also used as a hydration indicator for silica gel. Methyl violet 10B is also known as crystal violet and has medical uses.

Staining is a technique used to enhance contrast in samples, generally at the microscopic level. Stains and dyes are frequently used in histology and in the medical fields of histopathology, hematology, and cytopathology that focus on the study and diagnoses of disease at a microscopic level. Stains may be used to define biological tissues, cell populations, or organelles within individual cells.

Acid-fastness is a physical property of certain bacterial and eukaryotic cells, as well as some sub-cellular structures, specifically their resistance to decolorization by acids during laboratory staining procedures. Once stained as part of a sample, these organisms can resist the acid and/or ethanol-based decolorization procedures common in many staining protocols, hence the name acid-fast.
Fuchsine (sometimes spelled fuchsin) or rosaniline hydrochloride is a magenta dye with chemical formula C20H19N3·HCl. There are other similar chemical formulations of products sold as fuchsine, and several dozen other synonyms of this molecule.

New fuchsine is an organic compound with the formula [(H2N(CH3)C6H3)3C]Cl. It is a green-colored solid that is used as a dye of the triarylmethane class. It is one of the four components of basic fuchsine, and one of the two that are available as single dyes. The other is pararosaniline. It is prepared by condensation of ortho-toluidine with formaldehyde. This process initially gives the benzhydrol 4,4'-bis(dimethylamino)benzhydrol, which is further condensed to give the leuco (colorless) tertiary alcohol [(H2N(CH3)C6H3)3COH, which is oxidized in acid to give the dye.
Methyl blue is a chemical compound with the molecular formula C37H27N3Na2O9S3. It is used as a stain in histology, and stains collagen blue in tissue sections. It can be used in some differential staining techniques such as Mallory's connective tissue stain and Gömöri trichrome stain, and can be used to mediate electron transfer in microbial fuel cells. Fungal cell walls are also stained by methyl blue.

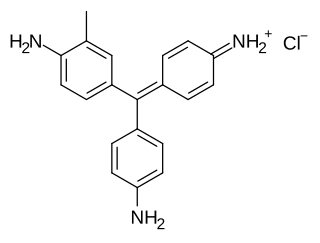
Crystal violet or gentian violet, also known as methyl violet 10B or hexamethyl pararosaniline chloride, is a triarylmethane dye used as a histological stain and in Gram's method of classifying bacteria. Crystal violet has antibacterial, antifungal, and anthelmintic (vermicide) properties and was formerly important as a topical antiseptic. The medical use of the dye has been largely superseded by more modern drugs, although it is still listed by the World Health Organization.
Acid dyes are anionic, soluble in water and are essentially applied from acidic bath. These dyes possess acidic groups, such as SO3H and COOH and are applied on wool, silk and nylon when ionic bond is established between protonated –NH2 group of fibre and acid group of dye. Overall wash fastness is poor although lightfastness is quite good. As dye and fibre contain opposite electrical nature, strike rate and uptake of acid dye on these fibres is faster; electrolyte at higher concentration is added to retard dye uptake and to form levelled shades. Acid generates cation on fibre and temperature helps to substitute negative part of acid with anionic dye molecules.
Trichrome staining is a histological staining method that uses two or more acid dyes in conjunction with a polyacid. Staining differentiates tissues by tinting them in contrasting colours. It increases the contrast of microscopic features in cells and tissues, which makes them easier to see when viewed through a microscope.

The Schiff test is an early organic chemistry named reaction developed by Hugo Schiff, and is a relatively general chemical test for detection of many organic aldehydes that has also found use in the staining of biological tissues. The Schiff reagent is the reaction product of a dye formulation such as fuchsin and sodium bisulfite; pararosaniline and new fuchsin are not dye alternatives with comparable detection chemistry.

Carbol fuchsin, carbol-fuchsin, or carbolfuchsin, is a mixture of phenol and basic fuchsin that is used in bacterial staining procedures. It is commonly used in the staining of mycobacteria because it has an affinity for the mycolic acids found in their cell membranes.
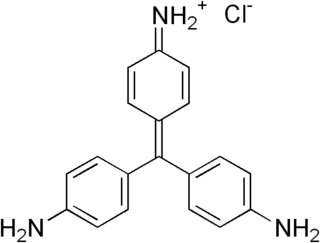
Pararosaniline, Basic Red 9, or C.I. 42500 is an organic compound with the formula [(H2NC6H4)3C]Cl. It is a magenta solid with a variety of uses as a dye. It is one of the four components of basic fuchsine. (The others are rosaniline, new fuchsine and magenta II.) It is structurally related to other triarylmethane dyes called methyl violets including crystal violet, which feature methyl groups on nitrogen.

Masson's trichrome is a three-colour staining protocol used in histology. The recipes evolved from Claude L. Pierre Masson's (1880–1959) original formulation have different specific applications, but all are suited for distinguishing cells from surrounding connective tissue.

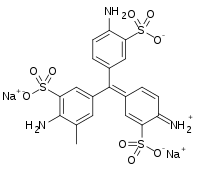
Water blue, also known as aniline blue, Acid blue 22, Soluble Blue 3M, Marine Blue V, or C.I. 42755, is a chemical compound used as a stain in histology. Water blue stains collagen blue in tissue sections. It is soluble in water and slightly soluble in ethanol.

Light green SF, also called C.I. 42095, light green SF yellowish, is a green triarylmethane dye.

Hematoxylin and eosin stain is one of the principal tissue stains used in histology. It is the most widely used stain in medical diagnosis and is often the gold standard. For example, when a pathologist looks at a biopsy of a suspected cancer, the histological section is likely to be stained with H&E.
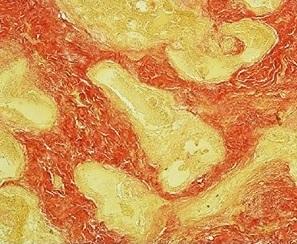
Van Gieson's stain is a mixture of picric acid and acid fuchsin. It is the simplest method of differential staining of collagen and other connective tissue. It was introduced to histology by American neuropsychiatrist and pathologist Ira Van Gieson.
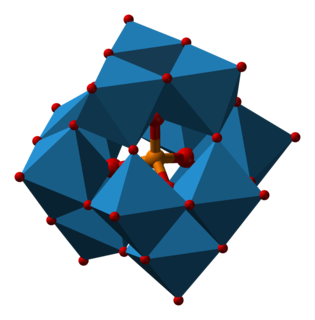
Phosphomolybdic acid is the heteropolymetalate with the formula H3Mo12PO40·12H2O. It is a yellow solid, although even slightly impure samples have a greenish coloration. It is also known as dodeca molybdophosphoric acid or PMA, is a yellow-green chemical compound that is freely soluble in water and polar organic solvents such as ethanol. It is used as a stain in histology and in organic synthesis.
Movat's stain is a pentachrome stain originally developed by Henry Zoltan Movat (1923–1995), a Hungarian-Canadian Pathologist in Toronto in 1955 to highlight the various constituents of connective tissue, especially cardiovascular tissue, by five colors in a single stained slide. In 1972, H. K. Russell, Jr. modified the technique so as to reduce the time for staining and to increase the consistency and reliability of the staining.