
Gram stain, is a method of staining used to classify bacterial species into two large groups: gram-positive bacteria and gram-negative bacteria. It may also be used to diagnose a fungal infection. The name comes from the Danish bacteriologist Hans Christian Gram, who developed the technique in 1884.

Haematoxylin or hematoxylin, also called natural black 1 or C.I. 75290, is a compound extracted from heartwood of the logwood tree with a chemical formula of C
16H
14O
6. This naturally derived dye has been used as a histologic stain, as an ink and as a dye in the textile and leather industry. As a dye, haematoxylin has been called palo de Campeche, logwood extract, bluewood and blackwood. In histology, haematoxylin staining is commonly followed by counterstaining with eosin. When paired, this staining procedure is known as H&E staining and is one of the most commonly used combinations in histology. In addition to its use in the H&E stain, haematoxylin is also a component of the Papanicolaou stain which is widely used in the study of cytology specimens.

Romanowsky staining is a prototypical staining technique that was the forerunner of several distinct but similar stains widely used in hematology and cytopathology. Romanowsky-type stains are used to differentiate cells for microscopic examination in pathological specimens, especially blood and bone marrow films, and to detect parasites such as malaria within the blood.
Mesitylene or 1,3,5-trimethylbenzene is a derivative of benzene with three methyl substituents positioned symmetrically around the ring. The other two isomeric trimethylbenzenes are 1,2,4-trimethylbenzene (pseudocumene) and 1,2,3-trimethylbenzene (hemimellitene). All three compounds have the formula C6H3(CH3)3, which is commonly abbreviated C6H3Me3. Mesitylene is a colorless liquid with sweet aromatic odor. It is a component of coal tar, which is its traditional source. It is a precursor to diverse fine chemicals. The mesityl group (Mes) is a substituent with the formula C6H2Me3 and is found in various other compounds.

Staining is a technique used to enhance contrast in samples, generally at the microscopic level. Stains and dyes are frequently used in histology, in cytology, and in the medical fields of histopathology, hematology, and cytopathology that focus on the study and diagnoses of diseases at the microscopic level. Stains may be used to define biological tissues, cell populations, or organelles within individual cells.

In biochemistry, immunostaining is any use of an antibody-based method to detect a specific protein in a sample. The term "immunostaining" was originally used to refer to the immunohistochemical staining of tissue sections, as first described by Albert Coons in 1941. However, immunostaining now encompasses a broad range of techniques used in histology, cell biology, and molecular biology that use antibody-based staining methods.
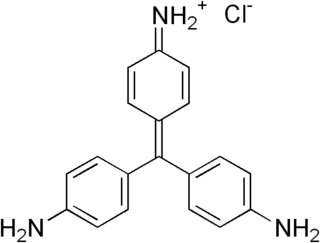
Fuchsine (sometimes spelled fuchsin) or rosaniline hydrochloride is a magenta dye with chemical formula C20H19N3·HCl. There are other similar chemical formulations of products sold as fuchsine, and several dozen other synonyms of this molecule.
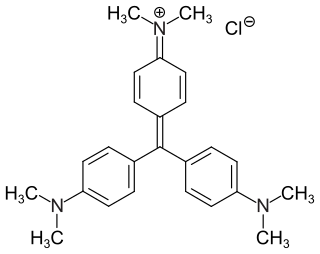
Crystal violet or gentian violet, also known as methyl violet 10B or hexamethyl pararosaniline chloride, is a triarylmethane dye used as a histological stain and in Gram's method of classifying bacteria. Crystal violet has antibacterial, antifungal, and anthelmintic (vermicide) properties and was formerly important as a topical antiseptic. The medical use of the dye has been largely superseded by more modern drugs, although it is still listed by the World Health Organization.
Trichrome staining is a histological staining method that uses two or more acid dyes in conjunction with a polyacid. Staining differentiates tissues by tinting them in contrasting colours. It increases the contrast of microscopic features in cells and tissues, which makes them easier to see when viewed through a microscope.

The Schiff test is an early organic chemistry named reaction developed by Hugo Schiff, and is a relatively general chemical test for detection of many organic aldehydes that has also found use in the staining of biological tissues. The Schiff reagent is the reaction product of a dye formulation such as fuchsin and sodium bisulfite; pararosaniline and new fuchsin are not dye alternatives with comparable detection chemistry.
Solvent Black 3 is an azo dye. It is a non-fluorescent, relatively thermostable lysochrome diazo dye used for staining of neutral triglycerides and lipids on frozen sections and some lipoproteins on paraffin sections. It has the appearance of a dark brown to black powder with maximum absorption at 596–605 nm and melting point 120–124 °C. It stains blue-black.
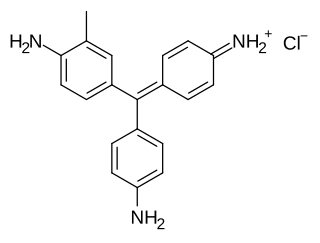
Pararosaniline, pararosaniline free base, Basic Red 9, or C.I. 42500 is an organic compound with the formula (H2NC6H4)2C=(C6H4NH). It is the leuco form of pararosaniline hydrochloride, [(H2NC6H4)3C]+Cl−, a magenta solid with a variety of uses as a dye. It is one of the four components of basic fuchsine. It is structurally related to other triarylmethane dyes called methyl violets which feature methyl groups on nitrogen.

Hematein or haematein is an oxidized derivative of haematoxylin, used in staining. Haematein should not be confused with haematin, which is a brown to black iron-containing pigment formed by decomposition of haemoglobin. In the Colour Index, haematein is called haematine.
Bismarck brown Y also called C.I. 21000 and C.I. Basic Brown 1, is a diazo dye with the idealized formula [(H2N)2C6H3N2]2C6H4. The dye is a mixture of closely related compounds. It was one of the earliest azo dyes, being described in 1863 by German chemist Carl Alexander von Martius. It is used in histology for staining tissues.

Hematoxylin and eosin stain is one of the principal tissue stains used in histology. It is the most widely used stain in medical diagnosis and is often the gold standard. For example, when a pathologist looks at a biopsy of a suspected cancer, the histological section is likely to be stained with H&E.
In the fields of histology, pathology, and cell biology, fixation is the preservation of biological tissues from decay due to autolysis or putrefaction. It terminates any ongoing biochemical reactions and may also increase the treated tissues' mechanical strength or stability. Tissue fixation is a critical step in the preparation of histological sections, its broad objective being to preserve cells and tissue components and to do this in such a way as to allow for the preparation of thin, stained sections. This allows the investigation of the tissues' structure, which is determined by the shapes and sizes of such macromolecules as proteins and nucleic acids.

Acid fuchsin or fuchsine acid, (also called Acid Violet 19 and C.I. 42685) is an acidic magenta dye with the chemical formula C20H17N3Na2O9S3. It is a sodium sulfonate derivative of fuchsine. Acid fuchsin has wide use in histology, and is one of the dyes used in Masson's trichrome stain. This method is commonly used to stain cytoplasm and nuclei of tissue sections in the histology laboratory in order to distinguish muscle from collagen. The muscle stains red with the acid fuchsin, and the collagen is stained green or blue with Light Green SF yellowish or methyl blue. It can also be used to identify growing bacteria.
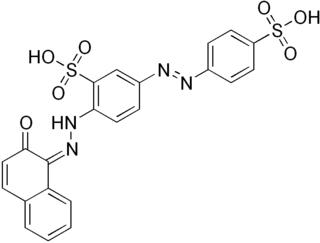
Biebrich scarlet is a molecule used in Lillie's trichrome. It is an anionic mono-azo dye, which is an important pigmenting agent in the textile and paper industries, used to color wool, silk, cotton, and papers. The dye was created in 1878 by the German chemist Rudolf Nietzki. He was employed by Kalle & Co. and completed his contributions on August of 1880, where he claimed to be the inventor of Biebrich scarlet. The name, Biebrich scarlet, originated from the location where a company, Kalle & Co., marketed the dye in Biebrich (Wiesbaden).
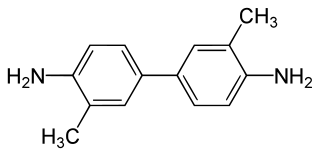
2-Tolidine (orthotolidine, o-tolidine; not to be confused with o-toluidine) is an organic compound with the chemical formula (C6H4(CH3)NH2)2. Several isomers are known; the 3-tolidine derivative is also important commercially. It is a colorless compound although commercial samples are often colored. It is slightly soluble in water. It forms salts with acids, such as the hydrochloride, which is commercially available.
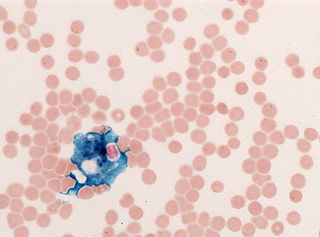
In histology, histopathology, and clinical pathology, Perls Prussian blue is a commonly used method to detect the presence of iron in tissue or cell samples. Perls Prussian Blue derives its name from the German pathologist Max Perls (1843–1881), who described the technique in 1867. The method does not involve the application of a dye, but rather causes the pigment Prussian blue to form directly within the tissue. The method stains mostly iron in the ferric state which includes ferritin and hemosiderin, rather than iron in the ferrous state.




















