
Analytical chemistry studies and uses instruments and methods to separate, identify, and quantify matter. In practice, separation, identification or quantification may constitute the entire analysis or be combined with another method. Separation isolates analytes. Qualitative analysis identifies analytes, while quantitative analysis determines the numerical amount or concentration.

An electrochemical cell is a device that generates electrical energy from chemical reactions. Electrical energy can also be applied to these cells to cause chemical reactions to occur. Electrochemical cells which generate an electric current are called voltaic or galvanic cells and those that generate chemical reactions, via electrolysis for example, are called electrolytic cells.

An electrolytic cell is an electrochemical cell that utilizes an external source of electrical energy to force a chemical reaction that would otherwise not occur. The external energy source is a voltage applied between the cell′s two electrodes; an anode and a cathode, which are immersed in an electrolyte solution. This is in contrast to a galvanic cell, which itself is a source of electrical energy and the foundation of a battery. The net reaction taking place in a galvanic cell is a spontaneous reaction, i.e, the Gibbs free energy remains -ve, while the net reaction taking place in an electrolytic cell is the reverse of this spontaneous reaction, i.e, the Gibbs free energy is +ve.

Gas chromatography (GC) is a common type of chromatography used in analytical chemistry for separating and analyzing compounds that can be vaporized without decomposition. Typical uses of GC include testing the purity of a particular substance, or separating the different components of a mixture. In preparative chromatography, GC can be used to prepare pure compounds from a mixture.
Analytical technique is a method used to determine a chemical or physical property of a chemical substance, chemical element, or mixture. There is a wide variety of techniques used for analysis, from simple weighing to advanced techniques using highly specialized instrumentation.
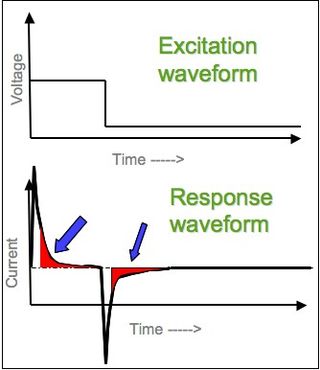
In electrochemistry, chronoamperometry is an analytical technique in which the electric potential of the working electrode is stepped and the resulting current from faradaic processes occurring at the electrode is monitored as a function of time. The functional relationship between current response and time is measured after applying single or double potential step to the working electrode of the electrochemical system. Limited information about the identity of the electrolyzed species can be obtained from the ratio of the peak oxidation current versus the peak reduction current. However, as with all pulsed techniques, chronoamperometry generates high charging currents, which decay exponentially with time as any RC circuit. The Faradaic current - which is due to electron transfer events and is most often the current component of interest - decays as described in the Cottrell equation. In most electrochemical cells, this decay is much slower than the charging decay-cells with no supporting electrolyte are notable exceptions. Most commonly a three-electrode system is used. Since the current is integrated over relatively longer time intervals, chronoamperometry gives a better signal-to-noise ratio in comparison to other amperometric techniques.
In analytical chemistry, potentiometric titration is a technique similar to direct titration of a redox reaction. It is a useful means of characterizing an acid. No indicator is used; instead the electric potential is measured across the analyte, typically an electrolyte solution. To do this, two electrodes are used, an indicator electrode and a reference electrode. Reference electrodes generally used are hydrogen electrodes, calomel electrodes, and silver chloride electrodes. The indicator electrode forms an electrochemical half-cell with the interested ions in the test solution. The reference electrode forms the other half-cell.
Electroanalytical methods are a class of techniques in analytical chemistry which study an analyte by measuring the potential (volts) and/or current (amperes) in an electrochemical cell containing the analyte. These methods can be broken down into several categories depending on which aspects of the cell are controlled and which are measured. The four main categories are potentiometry, amperometry, coulometry, and voltammetry.
Flow injection analysis (FIA) is an approach to chemical analysis. It is accomplished by injecting a plug of sample into a flowing carrier stream. The principle is similar to that of Segmented Flow Analysis (SFA) but no air is injected into the sample or reagent streams..

Christie G. Enke is a United States academic chemist who made pioneering contributions to the field of analytical chemistry.
Muhammad Rasul Jan is a Pakistani chemist in the field of analytical chemistry. He served as Vice-Chancellor of University of Malakand from 14 April 2008 till 1 October 2012. He also served as Vice-Chancellor at University of Peshawar and currently he is serving as Vice-Chancellor at the University of Poonch in Rawalakot, Azad Kashmir. He has published extensively in national and international journals and has supervised many M.Phil. and PhD scholars in the field of Analytical Chemistry.

Instrumental analysis is a field of analytical chemistry that investigates analytes using scientific instruments.
Amperometry in chemistry is detection of ions in a solution based on electric current or changes in electric current.
Jiří Barek is an electroanalytical chemist and university teacher of analytical chemistry. He graduated from Charles University, Faculty of Science, Department of Analytical Chemistry in 1972, and obtained his PhD in 1976 from the same institution. From 1977 to 1987 he served as a Lecturer, 1986–2006 as a Reader, and from 2006 he is a professor at the Department of Analytical Chemistry, Faculty of Science, Charles University, Prague. From 1993 to 1997 he served as the Deputy-Head and 2006–2012 as the Head of the Department of Analytical Chemistry, Charles University, Prague.
Debra R. Rolison is a physical chemist at the Naval Research Laboratory, where she is a head of the Advanced Electrochemical Materials section. Rolison's research involves the design, synthesis, and characterization of multi-functional nanostructures and ultra porous materials for rate-critical applications such as catalysis and energy storage. She is the 112th recipient of the William H. Nichols Medal Award.

Electrochemical stripping analysis is a set of analytical chemistry methods based on voltammetry or potentiometry that are used for quantitative determination of ions in solution. Stripping voltammetry have been employed for analysis of organic molecules as well as metal ions. Carbon paste, glassy carbon paste, and glassy carbon electrodes when modified are termed as chemically modified electrodes and have been employed for the analysis of organic and inorganic compounds.

Doron Aurbach is an Israeli electrochemist, materials and surface scientist.

Samuel Kounaves is an American researcher, academic and author. He is a Professor of Chemistry and adjunct Professor in Earth & Ocean Sciences at Tufts University. He also holds a visiting professorship at Imperial College London since 2014, a Consulting Scientist position at the Technical University of Berlin, and is an affiliate scientist at NASA’s Jet Propulsion Laboratory.










