Related Research Articles

A solvent is a substance that dissolves a solute, resulting in a solution. A solvent is usually a liquid but can also be a solid, a gas, or a supercritical fluid. Water is a solvent for polar molecules, and the most common solvent used by living things; all the ions and proteins in a cell are dissolved in water within the cell.

Solvations describes the interaction of a solvent with dissolved molecules. Both ionized and uncharged molecules interact strongly with a solvent, and the strength and nature of this interaction influence many properties of the solute, including solubility, reactivity, and color, as well as influencing the properties of the solvent such as its viscosity and density. If the attractive forces between the solvent and solute particles are greater than the attractive forces holding the solute particles together, the solvent particles pull the solute particles apart and surround them. The surrounded solute particles then move away from the solid solute and out into the solution. Ions are surrounded by a concentric shell of solvent. Solvation is the process of reorganizing solvent and solute molecules into solvation complexes and involves bond formation, hydrogen bonding, and van der Waals forces. Solvation of a solute by water is called hydration.
An electrolyte is a substance that conducts electricity through the movement of ions, but not through the movement of electrons. This includes most soluble salts, acids, and bases, dissolved in a polar solvent like water. Upon dissolving, the substance separates into cations and anions, which disperse uniformly throughout the solvent. Solid-state electrolytes also exist. In medicine and sometimes in chemistry, the term electrolyte refers to the substance that is dissolved.
In chemistry, hydronium is the cation [H3O]+, also written as H3O+, the type of oxonium ion produced by protonation of water. It is often viewed as the positive ion present when an Arrhenius acid is dissolved in water, as Arrhenius acid molecules in solution give up a proton to the surrounding water molecules. In fact, acids must be surrounded by more than a single water molecule in order to ionize, yielding aqueous H+ and conjugate base.

In chemistry, solubility is the ability of a substance, the solute, to form a solution with another substance, the solvent. Insolubility is the opposite property, the inability of the solute to form such a solution.

An aqueous solution is a solution in which the solvent is water. It is mostly shown in chemical equations by appending (aq) to the relevant chemical formula. For example, a solution of table salt, also known as sodium chloride (NaCl), in water would be represented as Na+(aq) + Cl−(aq). The word aqueous means pertaining to, related to, similar to, or dissolved in, water. As water is an excellent solvent and is also naturally abundant, it is a ubiquitous solvent in chemistry. Since water is frequently used as the solvent in experiments, the word solution refers to an aqueous solution, unless the solvent is specified.
The self-ionization of water (also autoionization of water, autoprotolysis of water, autodissociation of water, or simply dissociation of water) is an ionization reaction in pure water or in an aqueous solution, in which a water molecule, H2O, deprotonates (loses the nucleus of one of its hydrogen atoms) to become a hydroxide ion, OH−. The hydrogen nucleus, H+, immediately protonates another water molecule to form a hydronium cation, H3O+. It is an example of autoprotolysis, and exemplifies the amphoteric nature of water.
Revaz Dogonadze was a notable Georgian scientist, Corresponding Member of the Georgian National Academy of Sciences (GNAS) (1982), Doctor of Physical & Mathematical Sciences (1966), Professor (1972), one of the founders of quantum electrochemistry,

Liquid–liquid extraction, also known as solvent extraction and partitioning, is a method to separate compounds or metal complexes, based on their relative solubilities in two different immiscible liquids, usually water (polar) and an organic solvent (non-polar). There is a net transfer of one or more species from one liquid into another liquid phase, generally from aqueous to organic. The transfer is driven by chemical potential, i.e. once the transfer is complete, the overall system of chemical components that make up the solutes and the solvents are in a more stable configuration. The solvent that is enriched in solute(s) is called extract. The feed solution that is depleted in solute(s) is called the raffinate. Liquid–liquid extraction is a basic technique in chemical laboratories, where it is performed using a variety of apparatus, from separatory funnels to countercurrent distribution equipment called as mixer settlers. This type of process is commonly performed after a chemical reaction as part of the work-up, often including an acidic work-up.
Implicit solvation is a method to represent solvent as a continuous medium instead of individual “explicit” solvent molecules, most often used in molecular dynamics simulations and in other applications of molecular mechanics. The method is often applied to estimate free energy of solute-solvent interactions in structural and chemical processes, such as folding or conformational transitions of proteins, DNA, RNA, and polysaccharides, association of biological macromolecules with ligands, or transport of drugs across biological membranes.
In chemistry, the hydron, informally called proton, is the cationic form of atomic hydrogen, represented with the symbol H+
. The general term "hydron", endorsed by IUPAC, encompasses cations of hydrogen regardless of isotope: thus it refers collectively to protons (1H+) for the protium isotope, deuterons (2H+ or D+) for the deuterium isotope, and tritons (3H+ or T+) for the tritium isotope.
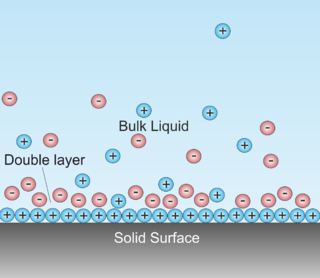
In surface science, a double layer is a structure that appears on the surface of an object when it is exposed to a fluid. The object might be a solid particle, a gas bubble, a liquid droplet, or a porous body. The DL refers to two parallel layers of charge surrounding the object. The first layer, the surface charge, consists of ions which are adsorbed onto the object due to chemical interactions. The second layer is composed of ions attracted to the surface charge via the Coulomb force, electrically screening the first layer. This second layer is loosely associated with the object. It is made of free ions that move in the fluid under the influence of electric attraction and thermal motion rather than being firmly anchored. It is thus called the "diffuse layer".
This glossary of chemistry terms is a list of terms and definitions relevant to chemistry, including chemical laws, diagrams and formulae, laboratory tools, glassware, and equipment. Chemistry is a physical science concerned with the composition, structure, and properties of matter, as well as the changes it undergoes during chemical reactions; it features an extensive vocabulary and a significant amount of jargon.

An ion is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convention. The net charge of an ion is not zero because its total number of electrons is unequal to its total number of protons.
The polarizable continuum model (PCM) is a commonly used method in computational chemistry to model solvation effects. If it is necessary to consider each solvent molecule as a separate molecule, the computational cost of modeling a solvent-mediated chemical reaction would grow prohibitively high. Modeling the solvent as a polarizable continuum, rather than individual molecules, makes ab initio computation feasible. Two types of PCMs have been popularly used: the dielectric PCM (D-PCM) in which the continuum is polarizable and the conductor-like PCM (C-PCM) in which the continuum is conductor-like similar to COSMO Solvation Model.

Bernd Michael Rode was an Austrian professor of chemistry at the University of Innsbruck and founder of the Austrian-South-East-Asian Academic University Network (ASEA-UNINET). Prof. Rode retired in 2011 but remained actively involved in teaching and research as well as in the thesis supervision.
In computational chemistry, a solvent model is a computational method that accounts for the behavior of solvated condensed phases. Solvent models enable simulations and thermodynamic calculations applicable to reactions and processes which take place in solution. These include biological, chemical and environmental processes. Such calculations can lead to new predictions about the physical processes occurring by improved understanding.

Biman Bagchi is an Indian scientist currently serving as a SERB-DST National Science Chair Professor and Honorary Professor at the Solid State and Structural Chemistry Unit of the Indian Institute of Science. He is a theoretical physical chemist and biophysicist known for his research in the area of statistical mechanics; particularly in the study of phase transition and nucleation, solvation dynamics, mode-coupling theory of electrolyte transport, dynamics of biological macromolecules, protein folding, enzyme kinetics, supercooled liquids and protein hydration layer. He is an elected fellow of the Indian National Science Academy, the Indian Academy of Sciences, The World Academy of Sciences and an International honorary member of the American Academy of Arts and Sciences. Along with several scientific articles, he has authored three books, (i) Molecular Relaxation in Liquids, (ii) Water in Biological and Chemical Processes: From Structure and Dynamics to Function, and (iii) Statistical Mechanics for Chemistry and Materials Science.

Klaas Wynne is a professor in the School of Chemistry at the University of Glasgow and chair of Chemical Physics. He was previously a professor in the Department of Physics at the University of Strathclyde (1996–2010).

Branka Maria Ladanyi was a Yugoslavian-born Croatian-American physical chemist, who spent her career in the department of chemistry at Colorado State University. Her research focused on structure and dynamics of liquids, broadly defined, which she studied using theoretical and computational techniques.
References
- 1 2 "Maroncelli Research Group | Experimental and Computational Solution Dynamics | Penn State University". April 13, 2012. Archived from the original on 2012-04-13.
- ↑ "Maroncelli Research Group | Experimental and Computational Solution Dynamics | Publications". April 24, 2014. Archived from the original on 2014-04-24.
- ↑ "Joe Henry Hidebrand Award report".
- ↑ "The Physical Chemistry Division of the American Chemical Society".
- ↑ "Humboldt Research Award Report".