
Reelin, encoded by the RELN gene, is a large secreted extracellular matrix glycoprotein that helps regulate processes of neuronal migration and positioning in the developing brain by controlling cell–cell interactions. Besides this important role in early development, reelin continues to work in the adult brain. It modulates synaptic plasticity by enhancing the induction and maintenance of long-term potentiation. It also stimulates dendrite and dendritic spine development and regulates the continuing migration of neuroblasts generated in adult neurogenesis sites like the subventricular and subgranular zones. It is found not only in the brain but also in the liver, thyroid gland, adrenal gland, fallopian tube, breast and in comparatively lower levels across a range of anatomical regions.
The development of the nervous system, or neural development (neurodevelopment), refers to the processes that generate, shape, and reshape the nervous system of animals, from the earliest stages of embryonic development to adulthood. The field of neural development draws on both neuroscience and developmental biology to describe and provide insight into the cellular and molecular mechanisms by which complex nervous systems develop, from nematodes and fruit flies to mammals.

Astrocytes, also known collectively as astroglia, are characteristic star-shaped glial cells in the brain and spinal cord. They perform many functions, including biochemical control of endothelial cells that form the blood–brain barrier, provision of nutrients to the nervous tissue, maintenance of extracellular ion balance, regulation of cerebral blood flow, and a role in the repair and scarring process of the brain and spinal cord following infection and traumatic injuries. The proportion of astrocytes in the brain is not well defined; depending on the counting technique used, studies have found that the astrocyte proportion varies by region and ranges from 20% to around 40% of all glia. Another study reports that astrocytes are the most numerous cell type in the brain. Astrocytes are the major source of cholesterol in the central nervous system. Apolipoprotein E transports cholesterol from astrocytes to neurons and other glial cells, regulating cell signaling in the brain. Astrocytes in humans are more than twenty times larger than in rodent brains, and make contact with more than ten times the number of synapses.
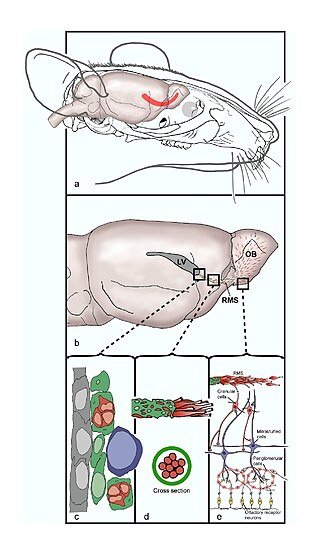
The rostral migratory stream (RMS) is a specialized migratory route found in the brain of some animals along which neuronal precursors that originated in the subventricular zone (SVZ) of the brain migrate to reach the main olfactory bulb (OB). The importance of the RMS lies in its ability to refine and even change an animal's sensitivity to smells, which explains its importance and larger size in the rodent brain as compared to the human brain, as our olfactory sense is not as developed. This pathway has been studied in the rodent, rabbit, and both the squirrel monkey and rhesus monkey. When the neurons reach the OB they differentiate into GABAergic interneurons as they are integrated into either the granule cell layer or periglomerular layer.

Radial glial cells, or radial glial progenitor cells (RGPs), are bipolar-shaped progenitor cells that are responsible for producing all of the neurons in the cerebral cortex. RGPs also produce certain lineages of glia, including astrocytes and oligodendrocytes. Their cell bodies (somata) reside in the embryonic ventricular zone, which lies next to the developing ventricular system.

Benjamin Arthur Barres was an American neurobiologist at Stanford University. His research focused on the interaction between neurons and glial cells in the nervous system. Beginning in 2008, he was chair of the Neurobiology Department at Stanford University School of Medicine. He transitioned to male in 1997, and became the first openly transgender scientist in the National Academy of Sciences in 2013.

Astrotactin-1, abbreviated ASTN1, is a glycoprotein expressed primarily in the central nervous system. ASTN1 and its counterpart ASTN2 are involved in regulation of adhesion during the radial migration of neurons in the developing CNS. Astrotactin is a neuronal adhesion molecule required for glial-guided migration of young postmitotic neuroblasts with expression is primarily located in the cells of cortical regions of the developing brain including; cerebrum, hippocampus, cerebellum, and olfactory bulb.

Ann Martin Graybiel is an Institute Professor and a faculty member in the Department of Brain and Cognitive Sciences at the Massachusetts Institute of Technology. She is also an investigator at the McGovern Institute for Brain Research. She is an expert on the basal ganglia and the neurophysiology of habit formation, implicit learning, and her work is relevant to Parkinson's disease, Huntington's disease, obsessive–compulsive disorder, substance abuse and other disorders that affect the basal ganglia.

T-box, brain, 1 is a transcription factor protein important in vertebrate embryo development. It is encoded by the TBR1 gene. This gene is also known by several other names: T-Brain 1, TBR-1, TES-56, and MGC141978. TBR1 is a member of the TBR1 subfamily of T-box family transcription factors, which share a common DNA-binding domain. Other members of the TBR1 subfamily include EOMES and TBX21. TBR1 is involved in the differentiation and migration of neurons and is required for normal brain development. TBR1 interacts with various genes and proteins in order to regulate cortical development, specifically within layer VI of the developing six-layered human cortex. Studies show that TBR1 may play a role in major neurological diseases such as Alzheimer's disease (AD), Parkinson's disease (PD) and autism spectrum disorder (ASD).

Gerald D. Fischbach is an American neuroscientist. He received his M.D. from the Weill Cornell Medical College of Cornell University in 1965 before beginning his research career at the National Institutes of Health in 1966, where his research focused on the mechanisms of neuromuscular junctions. After his tenure at the National Institutes of Health, Fischbach was a professor at Harvard University Medical School from 1972 to 1981 and from 1990 to 1998 and the Washington University School of Medicine from 1981 to 1990. In 1998, he was named the director of the National Institute of Neurological Disorders and Stroke before becoming the Vice President and Dean of the Health and Biomedical Sciences, the Dean of the Faculty of Medicine, and the Dean of the Faculty of Health Sciences at Columbia University from 2001 to 2006. Gerald Fischbach currently serves as the scientific director overseeing the Simons Foundation Autism Research Initiative. Throughout Fischbach's career, much of his research has focused on the formation and function of the neuromuscular junction, which stemmed from his innovative use of cell culture to study synaptic mechanisms.

Huda Yahya Zoghbi, born Huda El-Hibri, is a Lebanese-born American geneticist, and a professor at the Departments of Molecular and Human Genetics, Neuroscience and Neurology at the Baylor College of Medicine. She is the director of the Jan and Dan Duncan Neurological Research Institute. She was the editor of the Annual Review of Neuroscience from 2018-2024.
Nadine Gogolla is a Research Group Leader at the Max Planck Institute of Neurobiology in Martinsried, Germany as well as an Associate Faculty of the Graduate School for Systemic Neuroscience. Gogolla investigates the neural circuits underlying emotion to understand how the brain integrates external cues, feeling states, and emotions to make calculated behavioral decisions. Gogolla is known for her discovery using machine learning and two-photon microscopy to classify mouse facial expressions into emotion-like categories and correlate these facial expressions with neural activity in the insular cortex.
Anne Schaefer is a neuroscientist, professor of Neuroscience, vice-chair of Neuroscience, and director of the Center for Glial Biology at the Icahn School of Medicine at Mount Sinai in New York City. Schaefer investigates the epigenetic mechanisms of cellular plasticity and their role in the regulation of microglia-neuron interactions. Her research is aimed at understanding the mechanisms underlying various neuropsychiatric disorders and finding novel ways to target the epigenome therapeutically.

Gloria Choi is an American neuroscientist and neuroimmunologist and the Samuel A. Goldblith Career Development Professor in the Picower Institute for Learning and Memory at the Massachusetts Institute of Technology. Choi is known for elucidating the role of the immune system in the development of autism spectrum disorder-like phenotypes. Her lab currently explores how sensory experiences drive internal states and behavioural outcomes through probing the olfactory system as well as the neuroimmune system.
Camilla Bellone is an Italian neuroscientist and assistant professor in the Department of Basic Neuroscience at the University of Geneva, in Switzerland. Bellone's laboratory explores the molecular mechanisms and neural circuits underlying social behavior and probes how defects at the molecular and circuit level give rise to psychiatric disease states such as Autism Spectrum Disorders.

Katerina Akassoglou is a neuroimmunologist who is a Senior Investigator and Director of In Vivo Imaging Research at the Gladstone Institutes. Akassoglou holds faculty positions as a Professor of Neurology at the University of California, San Francisco. Akassoglou has pioneered investigations of blood-brain barrier integrity and development of neurological diseases. She found that compromised blood-brain barrier integrity leads to fibrinogen leakage into the brain inducing neurodegeneration. Akassoglou is internationally recognized for her scientific discoveries.
Lauren Orefice is an American neuroscientist and assistant professor in the Department of Molecular Biology at Massachusetts General Hospital and in the Department of Genetics at Harvard Medical School. Orefice has made innovative discoveries about the role of peripheral nerves and sensory hypersensitivity in the development of Autism-like behaviors. Her research now focuses on exploring the basic biology of somatosensory neural circuits for both touch and gastrointestinal function in order to shed light on how peripheral sensation impacts brain development and susceptibility to diseases like Autism Spectrum Disorders.
Mary Kay Lobo is an American psychiatric neuroscientist who is a Professor of Neurobiology at the University of Maryland School of Medicine. Her research considers the molecular mechanisms that underpin drug addiction and depression. She was named a finalist in the 2011 Blavatnik Awards for Young Scientists.
The pathophysiology of autism is the study of the physiological processes that cause or are otherwise associated with autism spectrum disorders.












