
An aerosol is a suspension of fine solid particles or liquid droplets, in air or another gas. Aerosols can be natural or anthropogenic. Examples of natural aerosols are fog, dust, forest exudates and geyser steam. Examples of anthropogenic aerosols are haze, particulate air pollutants and smoke. The liquid or solid particles have diameters typically <1 μm; larger particles with a significant settling speed make the mixture a suspension, but the distinction is not clear-cut. In general conversation, aerosol usually refers to an aerosol spray that delivers a consumer product from a can or similar container. Other technological applications of aerosols include dispersal of pesticides, medical treatment of respiratory illnesses, and convincing technology. Diseases can also spread by means of small droplets in the breath, also called aerosols.

Indoor air quality (IAQ) is the air quality within and around buildings and structures. IAQ is known to affect the health, comfort and well-being of building occupants. Poor indoor air quality has been linked to Sick Building Syndrome, reduced productivity and impaired learning in schools.

Ventilation is the intentional introduction of outdoor air into a space and is mainly used to control indoor air quality by diluting and displacing indoor pollutants; it can also be used for purposes of thermal comfort or dehumidification.

Renal function, in nephrology, is an indication of the kidney's condition and its role in renal physiology. Glomerular filtration rate (GFR) describes the flow rate of filtered fluid through the kidney. Creatinine clearance rate is the volume of blood plasma that is cleared of creatinine per unit time and is a useful measure for approximating the GFR. Creatinine clearance exceeds GFR due to creatinine secretion, which can be blocked by cimetidine. In alternative fashion, overestimation by older serum creatinine methods resulted in an underestimation of creatinine clearance, which provided a less biased estimate of GFR. Both GFR and CCr may be accurately calculated by comparative measurements of substances in the blood and urine, or estimated by formulas using just a blood test result.
Volatile organic compounds (VOCs) are organic chemicals that have a high vapor pressure at ordinary room temperature. Their high vapor pressure results from a low boiling point, which causes large numbers of molecules to evaporate or sublimate from the liquid or solid form of the compound and enter the surrounding air, a trait known as volatility. For example, formaldehyde, which evaporates from paint and releases from materials like resin, has a boiling point of only –19 °C (–2 °F).

An air quality index (AQI) is used by government agencies to communicate to the public how polluted the air currently is or how polluted it is forecast to become. As the AQI increases, an increasingly large percentage of the population is likely to experience increasingly severe adverse health effects. Different countries have their own air quality indices, corresponding to different national air quality standards. Some of these are the Air Quality Health Index (Canada), the Air Pollution Index (Malaysia), and the Pollutant Standards Index (Singapore).
In pharmacology, the clearance is a pharmacokinetic measurement of the volume of plasma from which a substance is completely removed per unit time; the usual units are mL/min. The quantity reflects the rate of drug elimination divided by plasma concentration.

Dilution is the process of decreasing the concentration of a solute in a solution, usually simply by mixing with more solvent like adding more water to a solution. To dilute a solution means to add more solvent without the addition of more solute. The resulting solution is thoroughly mixed so as to ensure that all parts of the solution are identical.
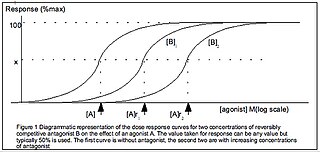
The dose–response relationship, or exposure–response relationship, describes the magnitude of the response of an organism, as a function of exposure to a stimulus or stressor after a certain exposure time. Dose–response relationships can be described by dose–response curves. This is explained further in the following sections. A stimulus response function or stimulus response curve is defined more broadly as the response from any type of stimulus, not limited to chemicals.
Various governmental agencies involved with environmental protection and with occupational safety and health have promulgated regulations limiting the allowable concentrations of gaseous pollutants in the ambient air or in emissions to the ambient air. Such regulations involve a number of different expressions of concentration. Some express the concentrations as ppmv and some express the concentrations as mg/m³, while others require adjusting or correcting the concentrations to reference conditions of moisture content, oxygen content or carbon dioxide content. This article presents a set of useful conversions and formulas for air dispersion modeling of atmospheric pollutants and for complying with the various regulations as to how to express the concentrations obtained by such modeling.

Total dissolved solids (TDS) is a measure of the dissolved combined content of all inorganic and organic substances present in a liquid in molecular, ionized or micro-granular suspended form. Generally the operational definition is that the solids must be small enough to survive filtration through a filter with two-micrometer pores. Total dissolved solids are normally discussed only for freshwater systems, as salinity includes some of the ions constituting the definition of TDS. The principal application of TDS is in the study of water quality for streams, rivers and lakes, although TDS is not generally considered a primary pollutant it is used as an indication of aesthetic characteristics of drinking water and as an aggregate indicator of the presence of a broad array of chemical contaminants.
Pharmacokinetics, sometimes abbreviated as PK, is a branch of pharmacology dedicated to determine the fate of substances administered to a living organism. The substances of interest include any chemical xenobiotic such as: pharmaceutical drugs, pesticides, food additives, cosmetics, etc. It attempts to analyze chemical metabolism and to discover the fate of a chemical from the moment that it is administered up to the point at which it is completely eliminated from the body. Pharmacokinetics is the study of how an organism affects a drug, whereas pharmacodynamics (PD) is the study of how the drug affects the organism. Both together influence dosing, benefit, and adverse effects, as seen in PK/PD models.
Turbulent diffusion is the transport of mass, heat, or momentum within a system due to random and chaotic time dependent motions. It occurs when turbulent fluid systems reach critical conditions in response to shear flow, which results from a combination of steep concentration gradients, density gradients, and high velocities. It occurs much more rapidly than molecular diffusion and is therefore extremely important for problems concerning mixing and transport in systems dealing with combustion, contaminants, dissolved oxygen, and solutions in industry. In these fields, turbulent diffusion acts as an excellent process for quickly reducing the concentrations of a species in a fluid or environment, in cases where this is needed for rapid mixing during processing, or rapid pollutant or contaminant reduction for safety.
Intake fraction is a measurement of pollution and it can be used in the determination of the environmental health impact of a pollutant source. Intake fraction is the ratio of the mass of a pollutant inhaled or ingested to the mass of the pollutant emitted. Traditionally, scientists and policy makers have used a source-oriented approach to reduce health impacts from pollution. That is, they identified the largest producers of various pollutants and worked to reduce the amount of pollutants emitted. Exposure assessment, also referred to as exposure analysis, is a receptor-oriented science, and practitioners in this field focus on the sources of pollutants that result in the largest human exposure. The same principles can be used to assess pollutant impact on non-human organisms, but most exposure analysts focus on humans.
The plateau principle is a mathematical model or scientific law originally developed to explain the time course of drug action. The principle has wide applicability in pharmacology, physiology, nutrition, biochemistry and system dynamics. It applies whenever a drug or nutrient is infused or ingested at a relatively constant rate and when a constant fraction is eliminated during each time interval. Under these conditions, any change in the rate of infusion leads to an exponential increase or decrease until a new level is achieved. This behavior is also called an approach to steady state because rather than causing an indefinite increase or decrease, a natural balance is achieved when the rate of infusion or production is balanced by the rate of loss.

The Streeter–Phelps equation is used in the study of water pollution as a water quality modelling tool. The model describes how dissolved oxygen (DO) decreases in a river or stream along a certain distance by degradation of biochemical oxygen demand (BOD). The equation was derived by H. W. Streeter, a sanitary engineer, and Earle B. Phelps, a consultant for the U.S. Public Health Service, in 1925, based on field data from the Ohio River. The equation is also known as the DO sag equation.
Bioconcentration is the accumulation of a chemical in or on an organism when the source of chemical is solely water. Bioconcentration is a term that was created for use in the field of aquatic toxicology. Bioconcentration can also be defined as the process by which a chemical concentration in an aquatic organism exceeds that in water as a result of exposure to a waterborne chemical.
Ott, W.R., Steinemann, A.C., Wallace, L.A.. Exposure Analysis. CRC Press (2007)
The Inside Story: A Guide to Indoor Air Quality. U.S. EPA (2009)














