Related Research Articles
The Nuremberg Code is a set of ethical research principles for human experimentation created by the court in U.S. v Brandt, one of the Subsequent Nuremberg trials that were held after the Second World War.
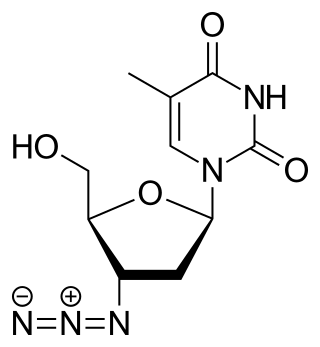
Zidovudine (ZDV), also known as azidothymidine (AZT), was the first antiretroviral medication used to prevent and treat HIV/AIDS. It is generally recommended for use in combination with other antiretrovirals. It may be used to prevent mother-to-child spread during birth or after a needlestick injury or other potential exposure. It is sold both by itself and together as lamivudine/zidovudine and abacavir/lamivudine/zidovudine. It can be used by mouth or by slow injection into a vein.

Clinical trials are prospective biomedical or behavioral research studies on human participants designed to answer specific questions about biomedical or behavioral interventions, including new treatments and known interventions that warrant further study and comparison. Clinical trials generate data on dosage, safety and efficacy. They are conducted only after they have received health authority/ethics committee approval in the country where approval of the therapy is sought. These authorities are responsible for vetting the risk/benefit ratio of the trial—their approval does not mean the therapy is 'safe' or effective, only that the trial may be conducted.

Human subject research is systematic, scientific investigation that can be either interventional or observational and involves human beings as research subjects, commonly known as test subjects. Human subject research can be either medical (clinical) research or non-medical research. Systematic investigation incorporates both the collection and analysis of data in order to answer a specific question. Medical human subject research often involves analysis of biological specimens, epidemiological and behavioral studies and medical chart review studies. On the other hand, human subject research in the social sciences often involves surveys which consist of questions to a particular group of people. Survey methodology includes questionnaires, interviews, and focus groups.

Trovafloxacin is a broad spectrum antibiotic that inhibits the uncoiling of supercoiled DNA in various bacteria by blocking the activity of DNA gyrase and topoisomerase IV. It was withdrawn from the market due to the risk of hepatotoxicity. It had better Gram-positive bacterial coverage but less Gram-negative coverage than the previous fluoroquinolones.

Henry Knowles Beecher was a pioneering American anesthesiologist, medical ethicist, and investigator of the placebo effect at Harvard Medical School.
The Kano trovafloxacin trial litigation arose out of a clinical trial conducted by the pharmaceutical company Pfizer in 1996 in Kano, Nigeria, during an epidemic of meningococcal meningitis. To test its new antibiotic, trovafloxacin (Trovan), Pfizer gave 100 children trovafloxacin, while another 100 received the gold-standard anti-meningitis treatment, ceftriaxone, a cephalosporin antibiotic. Pfizer gave the children a substantially reduced dose of the ceftriaxone relative to that described on the US FDA-approved prescribing information. The allegation is that this was done to skew the test in favor of its own drug. Pfizer claimed that the dose used was sufficient even though a clinical trial performed by Médecins Sans Frontières recommends a dose of 50–100 mg/kg.
The Stateville Penitentiary malaria study was a controlled but ethically questionable study of the effects of malaria on prisoners of Stateville Penitentiary near Joliet, Illinois, in the 1940s, conducted by the Department of Medicine at the University of Chicago in conjunction with the United States Army and the State Department. The Stateville experiment was viewed as coercive because it offered shortened sentences to participants. The Green report was written in 1945 about it by Andrew Conway Ivy, used in Nuremberg Medical Trial, which affected the Nuremberg Code, and used to discuss how medical experimentation on prisoners should be carried out.
An ethics committee is a body responsible for ensuring that medical experimentation and human subject research are carried out in an ethical manner in accordance with national and international law.

Nazi human experimentation was a series of medical experiments on prisoners by Nazi Germany in its concentration camps mainly between 1942 and 1945. There were 15,754 documented victims, of various nationalities and age groups, although the true number is believed to be more extensive. Many survived, with a quarter of documented victims being killed. Survivors generally experienced severe permanent injuries.

Medical Apartheid: The Dark History of Medical Experimentation on Black Americans from Colonial Times to the Present is a 2007 book by Harriet A. Washington. It is a history of medical experimentation on African Americans. From the era of slavery to the present day, this book presents the first detailed account of black Americans' abuse as unwitting subjects of medical experimentation.

Medroxyprogesterone acetate (MPA), also known as depot medroxyprogesterone acetate (DMPA) in injectable form and sold under the brand name Depo-Provera among others, is a hormonal medication of the progestin type. It is used as a method of birth control and as a part of menopausal hormone therapy. It is also used to treat endometriosis, abnormal uterine bleeding, paraphilia, and certain types of cancer. The medication is available both alone and in combination with an estrogen. It is taken by mouth, used under the tongue, or by injection into a muscle or fat.

Claus Karl Schilling, also recorded as Klaus Schilling, was a German tropical medicine specialist who participated in the Nazi human experiments at the Dachau concentration camp during World War II.

Numerous experiments which were performed on human test subjects in the United States in the past are now considered to have been unethical, because they were performed without the knowledge or informed consent of the test subjects. Such tests have been performed throughout American history, but have become significantly less frequent with the advent and adoption of various safeguarding efforts. Despite these safeguards, unethical experimentation involving human subjects is still occasionally uncovered.
Human subject research legislation in the United States can be traced to the early 20th century. Human subject research in the United States was mostly unregulated until the 20th century, as it was throughout the world, until the establishment of various governmental and professional regulations and codes of ethics. Notable – and in some cases, notorious – human subject experiments performed in the US include the Tuskegee syphilis experiment, human radiation experiments, the Milgram obedience experiment and Stanford prison experiments and Project MKULTRA. With growing public awareness of such experimentation, and the evolution of professional ethical standards, such research became regulated by various legislation, most notably, those that introduced and then empowered the institutional review boards.
The term informed assent describes the process whereby minors may agree to participate in clinical trials. It is similar to the process of informed consent in adults, however there remains some overlap between the terms.
Various organizations have created guidelines for human subject research for various kinds of research involving human subjects and for various situations.

Harriet A. Washington is an American writer and medical ethicist. She is the author of the book Medical Apartheid, which won the 2007 National Book Critics Circle Award for Nonfiction. She has also written books on environmental racism and the erosion of informed consent in medicine.
Unethical human experimentation is human experimentation that violates the principles of medical ethics. Such practices have included denying patients the right to informed consent, using pseudoscientific frameworks such as race science, and torturing people under the guise of research. Around World War II, Imperial Japan and Nazi Germany carried out brutal experiments on prisoners and civilians through groups like Unit 731 or individuals like Josef Mengele; the Nuremberg Code was developed after the war in response to the Nazi experiments. Countries have carried out brutal experiments on marginalized populations. Examples include American abuses during Project MKUltra and the Tuskegee syphilis experiments, and the mistreatment of indigenous populations in Canada and Australia. The Declaration of Helsinki, developed by the World Medical Association (WMA), is widely regarded as the cornerstone document on human research ethics.
References
- 1 2 3 Washington, Harriet A. Medical Apartheid , Anchor Books 2006 p390
- 1 2 3 4 Washington, Harriet A. Medical Apartheid , Anchor Books 2006 p392-393
- ↑ "Africa | Nigeria sues drugs giant Pfizer". BBC News . 2007-06-05. Retrieved 2010-11-12.
- 1 2 3 4 5 6 7 8 Meier, Benjamin Mason: International Protection of Persons Undergoing Medical Experimentation: Protecting the Right of Informed Consent, Berkeley Journal of International Law [1085-5718] Meier yr:2002 vol:20 iss:3 pg:513 -554
- 1 2 Kaler, Amy. 1998. "A Threat to the Nation and a Threat to the Men: the Banning of Depo-Provera in Zimbabwe, 1981". Journal of Southern African Studies 24(2):p 347
- ↑ "Herero and Namaqua Genocide - Herero Genocide Nama Genocide". Archived from the original on 2011-12-09. Retrieved 2011-11-28.
- ↑ Eugen Fischer
- 1 2 Washington, Harriet A. (2007-07-31). "Why Africa Fears Western Medicine". The New York Times.
- ↑ Washington, Harriet A. Medical Apartheid , Anchor Books 2006 p394
- ↑ Krosin, Michael T.; Klitzman, Robert; Levin, Bruce; Cheng, Jianfeng; Ranney, Megan L. (2006-06-01). "Problems in comprehension of informed consent in rural and peri-urban Mali, West Africa — Clinical Trials". Clinical Trials. 3 (3). Ctj.sagepub.com: 306–313. doi:10.1191/1740774506cn150oa. PMID 16895047. S2CID 24491268 . Retrieved 2010-11-12.
- ↑ "allAfrica.com: Africa: Women And Scientific Experiments - Is Informed Consent Enough? (Page 1 of 3)". Archived from the original on 2008-05-31.