
Drosophila melanogaster is a species of fly in the family Drosophilidae. The species is often referred to as the fruit fly or lesser fruit fly, however its common name is more accurately the vinegar fly. Starting with Charles W. Woodworth's proposal of the use of this species as a model organism, D. melanogaster continues to be widely used for biological research in genetics, physiology, microbial pathogenesis, and life history evolution. As of 2017, five Nobel Prizes have been awarded to [drosophilists] for their work using the animal.

Toll-like receptors (TLRs) are a class of proteins that play a key role in the innate immune system. They are single-pass membrane-spanning receptors usually expressed on sentinel cells such as macrophages and dendritic cells, that recognize structurally conserved molecules derived from microbes. Once these microbes have breached physical barriers such as the skin or intestinal tract mucosa, they are recognized by TLRs, which activate immune cell responses. The TLRs include TLR1, TLR2, TLR3, TLR4, TLR5, TLR6, TLR7, TLR8, TLR9, TLR10, TLR11, TLR12, and TLR13, though the last three are not found in humans, and there isn't a functional gene for TLR10 in mice. TLR1, TLR2, TLR4, TLR5, TLR6, and TLR10 are located on the cell membrane, whereas TLR3, TLR7, TLR8, and TLR9 are located in intracellular vesicles.

Defensins are small cysteine-rich cationic proteins across cellular life, including vertebrate and invertebrate animals, plants, and fungi. They are host defense peptides, with members displaying either direct antimicrobial activity, immune signalling activities, or both. They are variously active against bacteria, fungi and many enveloped and nonenveloped viruses. They are typically 18-45 amino acids in length, with three or four highly conserved disulphide bonds.

Providencia is genus of Gram-negative, motile bacteria of the family Morganellaceae. It was named after Providence, Rhode Island, where C. A. Stuart and colleagues studied these bacteria at Brown University.

Cathelicidin antimicrobial peptide (CAMP) is a polypeptide that is primarily stored in the lysosomes of macrophages and polymorphonuclear leukocytes (PMNs); in humans, the CAMP gene encodes the peptide precursor CAP-18, which is processed by proteinase 3-mediated extracellular cleavage into the active form LL-37. LL-37 is the only peptide in the Cathelicidin family found in the human body.
Period (per) is a gene located on the X chromosome of Drosophila melanogaster. Oscillations in levels of both per transcript and its corresponding protein PER have a period of approximately 24 hours and together play a central role in the molecular mechanism of the Drosophila biological clock driving circadian rhythms in eclosion and locomotor activity. Mutations in the per gene can shorten (perS), lengthen (perL), and even abolish (per0) the period of the circadian rhythm.

Jules A. Hoffmann is a Luxembourg-born French biologist. During his youth, growing up in Luxembourg, he developed a strong interest in insects under the influence of his father, Jos Hoffmann. This eventually resulted in the younger Hoffmann's dedication to the field of biology using insects as model organisms. He currently holds a faculty position at the University of Strasbourg. He is a research director and member of the board of administrators of the National Center of Scientific Research (CNRS) in Strasbourg, France. He was elected to the positions of Vice-President (2005-2006) and President (2007-2008) of the French Academy of Sciences. Hoffmann and Bruce Beutler were jointly awarded a half share of the 2011 Nobel Prize in Physiology or Medicine for "their discoveries concerning the activation of innate immunity,". [More specifically, the work showing increased Drosomycin expression following activation of Toll pathway in microbial infection.]

Arthropod defensins are a family defensin proteins found in mollusks, insects, and arachnids. These cysteine-rich antibacterial peptides are primarily active against Gram-positive bacteria and fungi in vitro. However Drosophila fruit flies mutant for the fly defensin were more susceptible to infection by the Gram-negative bacteria Providencia burhodogranariea, and resisted infection against Gram-positive bacteria like wild-type flies. It remains to be seen how in vitro activity relates to in vivo function. Mutants for the defensin-like antimicrobial peptide Drosomycin were more susceptible to fungi, validating a role for defensin-like peptides in anti-fungal defence.

Pleuromutilin and its derivatives are antibacterial drugs that inhibit protein synthesis in bacteria by binding to the peptidyl transferase component of the 50S subunit of ribosomes.
Thioester containing protein 1, often simply referred to as TEP1 is a key component of the arthropod innate immune system. TEP1 was first identified as a key immunity gene in 2001 through functional studies on Anopheles gambiae mosquitoes.
The microbiota describes the sum of all symbiotic microorganisms living on or in an organism. The fruit fly Drosophila melanogaster is a model organism and known as one of the most investigated organisms worldwide. The microbiota in flies is less complex than that found in humans. It still has an influence on the fitness of the fly, and it affects different life-history characteristics such as lifespan, resistance against pathogens (immunity) and metabolic processes (digestion). Considering the comprehensive toolkit available for research in Drosophila, analysis of its microbiome could enhance our understanding of similar processes in other types of host-microbiota interactions, including those involving humans. Microbiota plays key roles in the intestinal immune and metabolic responses via their fermentation product, acetate.

Drosomycin is an antifungal peptide from Drosophila melanogaster and was the first antifungal peptide isolated from insects. Drosomycin is induced by infection by the Toll signalling pathway, while expression in surface epithelia like the respiratory tract is instead controlled by the immune deficiency pathway (Imd). This means that drosomycin, alongside other antimicrobial peptides (AMPs) such as cecropins, diptericin, drosocin, metchnikowin and attacin, serves as a first line defence upon septic injury. However drosomycin is also expressed constitutively to a lesser extent in different tissues and throughout development.

Drosophila neotestacea is a member of the testacea species group of Drosophila. Testacea species are specialist fruit flies that breed on the fruiting bodies of mushrooms. These flies will choose to breed on psychoactive mushrooms such as the Fly Agaric Amanita muscaria. Drosophila neotestacea can be found in temperate regions of North America, ranging from the north eastern United States to western Canada.

Diptericin is a 9 kDa antimicrobial peptide (AMP) of flies first isolated from the blowfly Phormia terranova. It is primarily active against Gram-negative bacteria, disrupting bacterial membrane integrity. The structure of this protein includes a proline-rich domain with similarities to the AMPs drosocin, pyrrhocoricin, and abaecin, and a glycine-rich domain with similarity to attacin. Diptericin is an iconic readout of immune system activity in flies, used ubiquitously in studies of Drosophila immunity. Diptericin is named after the insect order Diptera.

Drosocin is a 19-residue long antimicrobial peptide (AMP) of flies first isolated in the fruit fly Drosophila melanogaster, and later shown to be conserved throughout the genus Drosophila. Drosocin is regulated by the NF-κB Imd signalling pathway in the fly.

Pyrrhocoricin is a 20-residue long antimicrobial peptide of the firebug Pyrrhocoris apterus.

The Drosophila quinaria species group is a speciose lineage of mushroom-feeding flies studied for their specialist ecology, their parasites, population genetics, and the evolution of immune systems. Quinaria species are part of the Drosophila subgenus.

Drosophila innubila is a species of vinegar fly restricted to high-elevation woodlands in the mountains of the southern USA and Mexico, which it likely colonized during the last glacial period. Drosophila innubila is a kind of mushroom-breeding Drosophila, and member of the Drosophila quinaria species group. Drosophila innubila is best known for its association with a strain of male-killing Wolbachia bacteria. These bacteria are parasitic, as they drain resources from the host and cause half the infected female's eggs to abort. However Wolbachia may offer benefits to the fly's fitness in certain circumstances. The D. innubila genome was sequenced in 2019.
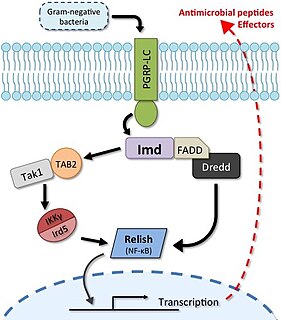
The Imd pathway is a broadly-conserved NF-κB immune signalling pathway of insects and some arthropods that regulates a potent antibacterial defence response. The pathway is named after the discovery of a mutation causing severe immune deficiency. The Imd pathway was first discovered in 1995 using Drosophila fruit flies by Bruno Lemaitre and colleagues, who also later discovered that the Drosophila Toll gene regulated defence against Gram-positive bacteria and fungi. Together the Toll and Imd pathways have formed a paradigm of insect immune signalling; as of September 2, 2019, these two landmark discovery papers have been cited collectively over 5000 times since publication on Google Scholar.

Bruno Lemaitre is a French immunologist and a professor at the École Polytechnique Fédérale de Lausanne (EPFL). His research focuses on the mechanisms of innate immunity and endosymbiosis in Drosophila. Lemaitre has also authored several books on the topic of narcissism in science.















