Related Research Articles

Combustion, or burning, is a high-temperature exothermic redox chemical reaction between a fuel and an oxidant, usually atmospheric oxygen, that produces oxidized, often gaseous products, in a mixture termed as smoke. Combustion does not always result in fire, because a flame is only visible when substances undergoing combustion vaporize, but when it does, a flame is a characteristic indicator of the reaction. While activation energy must be supplied to initiate combustion, the heat from a flame may provide enough energy to make the reaction self-sustaining.
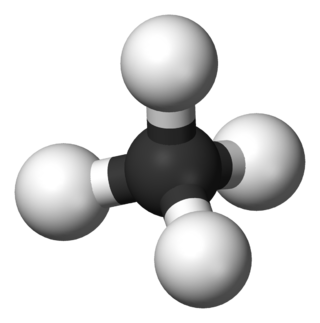
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and hydrophobic; their odor is usually faint, and may be similar to that of gasoline or lighter fluid. They occur in a diverse range of molecular structures and phases: they can be gases, liquids, low melting solids or polymers.

Propane is a three-carbon alkane with the molecular formula C3H8. It is a gas at standard temperature and pressure, but compressible to a transportable liquid. A by-product of natural gas processing and petroleum refining, it is commonly used as a fuel in domestic and industrial applications and in low-emissions public transportation. Discovered in 1857 by the French chemist Marcellin Berthelot, it became commercially available in the US by 1911. Propane is one of a group of liquefied petroleum gases. The others include butane, propylene, butadiene, butylene, isobutylene, and mixtures thereof. Propane has lower volumetric energy density, but higher gravimetric energy density and burns more cleanly than gasoline and coal.

Ethane is an organic chemical compound with chemical formula C
2H
6. At standard temperature and pressure, ethane is a colorless, odorless gas. Like many hydrocarbons, ethane is isolated on an industrial scale from natural gas and as a petrochemical by-product of petroleum refining. Its chief use is as feedstock for ethylene production.

Liquefied petroleum gas is a fuel gas which contains a flammable mixture of hydrocarbon gases, specifically propane, propylene, butylene, isobutane, and n-butane.
Dimethyl ether (DME; also known as methoxymethane) is the organic compound with the formula CH3OCH3, (sometimes ambiguously simplified to C2H6O as it is an isomer of ethanol). The simplest ether, it is a colorless gas that is a useful precursor to other organic compounds and an aerosol propellant that is currently being demonstrated for use in a variety of fuel applications.
Natural-gas condensate, also called natural gas liquids, is a low-density mixture of hydrocarbon liquids that are present as gaseous components in the raw natural gas produced from many natural gas fields. Some gas species within the raw natural gas will condense to a liquid state if the temperature is reduced to below the hydrocarbon dew point temperature at a set pressure.

Propyne (methylacetylene) is an alkyne with the chemical formula CH3C≡CH. It is a component of MAPD gas—along with its isomer propadiene (allene), which was commonly used in gas welding. Unlike acetylene, propyne can be safely condensed.
Propylene, also known as propene, is an unsaturated organic compound with the chemical formula CH3CH=CH2. It has one double bond, and is the second simplest member of the alkene class of hydrocarbons. It is a colorless gas with a faint petroleum-like odor.

MAPP gas was a trademarked name, belonging to The Linde Group, a division of the former global chemical giant Union Carbide, for a fuel gas based on a stabilized mixture of methylacetylene (propyne), propadiene and propane. The name comes from the original chemical composition, methylacetylene-propadiene propane. "MAPP gas" is also widely used as a generic name for UN 1060 stabilised methylacetylene-propadiene.

Fuel gas is any one of a number of fuels that under ordinary conditions are gaseous. Most fuel gases are composed of hydrocarbons, hydrogen, carbon monoxide, or mixtures thereof. Such gases are sources of energy that can be readily transmitted and distributed through pipes.

Autogas or LPG is liquefied petroleum gas (LPG) used as a fuel in internal combustion engines in vehicles as well as in stationary applications such as generators. It is a mixture of propane and butane.

Industrial gases are the gaseous materials that are manufactured for use in industry. The principal gases provided are nitrogen, oxygen, carbon dioxide, argon, hydrogen, helium and acetylene, although many other gases and mixtures are also available in gas cylinders. The industry producing these gases is also known as industrial gas, which is seen as also encompassing the supply of equipment and technology to produce and use the gases. Their production is a part of the wider chemical Industry.

A propane torch is a tool normally used for the application of flame or heat which uses propane, a hydrocarbon gas, for its fuel and ambient air as its combustion medium. Propane is one of a group of by-products of the natural gas and petroleum industries known as liquefied petroleum gas (LPG). Propane and other fuel torches are most commonly used in the manufacturing, construction and metal-working industries.

Oxy-fuel welding and oxy-fuel cutting are processes that use fuel gases and oxygen to weld or cut metals. French engineers Edmond Fouché and Charles Picard became the first to develop oxygen-acetylene welding in 1903. Pure oxygen, instead of air, is used to increase the flame temperature to allow localised melting of the workpiece material in a room environment. A common propane/air flame burns at about 2,250 K, a propane/oxygen flame burns at about 2,526 K, an oxyhydrogen flame burns at 3,073 K and an acetylene/oxygen flame burns at about 3,773 K.
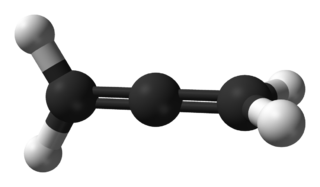
Propadiene or allene is the organic compound with the formula H2C=C=CH2. It is the simplest allene, i.e. a compound with two adjacent carbon double bonds. As a constituent of MAPP gas, it has been used as a fuel for specialized welding.

A fuel is any material that can be made to react with other substances so that it releases energy as thermal energy or to be used for work. The concept was originally applied solely to those materials capable of releasing chemical energy but has since also been applied to other sources of heat energy, such as nuclear energy.

Butane or n-butane is an alkane with the formula C4H10. Butane is a highly flammable, colorless, easily liquefied gas that quickly vaporizes at room temperature and pressure. The name butane comes from the root but- (from butyric acid, named after the Greek word for butter) and the suffix -ane. It was discovered in crude petroleum in 1864 by Edmund Ronalds, who was the first to describe its properties, and commercialized by Walter O. Snelling in early 1910s.

A blowtorch, also referred to as a blowlamp, is an ambient air fuel-burning gas lamp used for applying flame and heat to various applications, usually metalworking.

Steam cracking is a petrochemical process in which saturated hydrocarbons are broken down into smaller, often unsaturated, hydrocarbons. It is the principal industrial method for producing the lighter alkenes, including ethene and propene. Steam cracker units are facilities in which a feedstock such as naphtha, liquefied petroleum gas (LPG), ethane, propane or butane is thermally cracked through the use of steam in steam cracking furnaces to produce lighter hydrocarbons. The propane dehydrogenation process may be accomplished through different commercial technologies. The main differences between each of them concerns the catalyst employed, design of the reactor and strategies to achieve higher conversion rates.
References
- 1 2 Jeffus, Larry (January 23, 2020). Welding: Principles and Applications. Cengage Learning. p. 172. ISBN 9780357377802 – via Google Books.
- ↑ Porter, Michael (2004). Gas Burners for Forges, Furnaces, and Kilns. City: SkipJack Press. ISBN 1-879535-20-3.