
Coke is a grey, hard, and porous coal-based fuel with a high carbon content and few impurities, made by heating coal or oil in the absence of air—a destructive distillation process. It is an important industrial product, used mainly in iron ore smelting, but also as a fuel in stoves and forges when air pollution is a concern.

Producer gas is fuel gas that is manufactured by blowing through a coke or coal fire with air and steam simultaneously. It mainly consists of carbon monoxide (CO), hydrogen (H2), as well as substantial amounts of nitrogen (N2). The caloric value of the producer gas is low (mainly because of its high nitrogen content), and the technology is obsolete. Improvements over producer gas, also obsolete, include water gas where the solid fuel is treated intermittently with air and steam and, far more efficiently synthesis gas where the solid fuel is replaced with methane.
Syngas, or synthesis gas, is a mixture of hydrogen and carbon monoxide, in various ratios. The gas often contains some carbon dioxide and methane. It is principally used for producing ammonia or methanol. Syngas is combustible and can be used as a fuel. Historically, it has been used as a replacement for gasoline, when gasoline supply has been limited; for example, wood gas was used to power cars in Europe during WWII.

The pyrolysis process is the thermal decomposition of materials at elevated temperatures, often in an inert atmosphere. The word is coined from the Greek-derived elements pyro "fire", "heat", "fever" and lysis "separating".

Gasification is a process that converts biomass- or fossil fuel-based carbonaceous materials into gases, including as the largest fractions: nitrogen (N2), carbon monoxide (CO), hydrogen (H2), and carbon dioxide (CO2). This is achieved by reacting the feedstock material at high temperatures (typically >700 °C), without combustion, via controlling the amount of oxygen and/or steam present in the reaction. The resulting gas mixture is called syngas (from synthesis gas) or producer gas and is itself a fuel due to the flammability of the H2 and CO of which the gas is largely composed. Power can be derived from the subsequent combustion of the resultant gas, and is considered to be a source of renewable energy if the gasified compounds were obtained from biomass feedstock.

Liquefied petroleum gas is a fuel gas which contains a flammable mixture of hydrocarbon gases, specifically propane, propylene, butylene, isobutane, and n-butane.
Coal gas is a flammable gaseous fuel made from coal and supplied to the user via a piped distribution system. It is produced when coal is heated strongly in the absence of air. Town gas is a more general term referring to manufactured gaseous fuels produced for sale to consumers and municipalities.
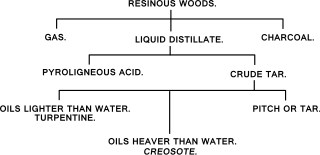
Dry distillation is the heating of solid materials to produce gaseous products. The method may involve pyrolysis or thermolysis, or it may not.
The heating value of a substance, usually a fuel or food, is the amount of heat released during the combustion of a specified amount of it.
The Fischer–Tropsch process (FT) is a collection of chemical reactions that converts a mixture of carbon monoxide and hydrogen, known as syngas, into liquid hydrocarbons. These reactions occur in the presence of metal catalysts, typically at temperatures of 150–300 °C (302–572 °F) and pressures of one to several tens of atmospheres. The Fischer–Tropsch process is an important reaction in both coal liquefaction and gas to liquids technology for producing liquid hydrocarbons.
In industrial chemistry, coal gasification is the process of producing syngas—a mixture consisting primarily of carbon monoxide (CO), hydrogen, carbon dioxide, methane, and water vapour —from coal and water, air and/or oxygen.
The Wobbe index (WI) or Wobbe number is an indicator of the interchangeability of fuel gases such as natural gas, liquefied petroleum gas (LPG), and town gas and is frequently defined in the specifications of gas supply and transport utilities.
Coal liquefaction is a process of converting coal into liquid hydrocarbons: liquid fuels and petrochemicals. This process is often known as "Coal to X" or "Carbon to X", where X can be many different hydrocarbon-based products. However, the most common process chain is "Coal to Liquid Fuels" (CTL).

Synthetic fuel or synfuel is a liquid fuel, or sometimes gaseous fuel, obtained from syngas, a mixture of carbon monoxide and hydrogen, in which the syngas was derived from gasification of solid feedstocks such as coal or biomass or by reforming of natural gas.
Water gas is a kind of fuel gas, a mixture of carbon monoxide and hydrogen. It is produced by "alternately hot blowing a fuel layer [coke] with air and gasifying it with steam". The caloric yield of this is about 10% of a modern syngas plant. Further making this technology unattractive, its precursor coke is expensive, whereas syngas uses cheaper precursor, mainly methane from natural gas.

The Karrick process is a low-temperature carbonization (LTC) and pyrolysis process of carbonaceous materials. Although primarily meant for coal carbonization, it also could be used for processing of oil shale, lignite or any carbonaceous materials. These are heated at 450 °C (800 °F) to 700 °C (1,300 °F) in the absence of air to distill out synthetic fuels–unconventional oil and syngas. It could be used for a coal liquefaction as also for a semi-coke production. The process was the work of oil shale technologist Lewis Cass Karrick at the United States Bureau of Mines in the 1920s.
Pyrolysis oil, sometimes also known as bio-crude or bio-oil, is a synthetic fuel under investigation as substitute for petroleum. It is obtained by heating dried biomass without oxygen in a reactor at a temperature of about 500 °C (900 °F) with subsequent cooling. Pyrolysis oil is a kind of tar and normally contains levels of oxygen too high to be considered a pure hydrocarbon. This high oxygen content results in non-volatility, corrosiveness, immiscibility with fossil fuels, thermal instability, and a tendency to polymerize when exposed to air. As such, it is distinctly different from petroleum products. Removing oxygen from bio-oil or nitrogen from algal bio-oil is known as upgrading.
Renewable Fuels are fuels produced from renewable resources. Examples include: biofuels, Hydrogen fuel, and fully synthetic fuel produced from ambient carbon dioxide and water. This is in contrast to non-renewable fuels such as natural gas, LPG (propane), petroleum and other fossil fuels and nuclear energy. Renewable fuels can include fuels that are synthesized from renewable energy sources, such as wind and solar. Renewable fuels have gained in popularity due to their sustainability, low contributions to the carbon cycle, and in some cases lower amounts of greenhouse gases. The geo-political ramifications of these fuels are also of interest, particularly to industrialized economies which desire independence from Middle Eastern oil.
Oil shale gas is a synthetic non-condensable gas mixture (syngas) produced by oil shale thermal processing (pyrolysis). Although often referred to as shale gas, it differs from the natural gas produced from shale, which is also known as shale gas.










