Related Research Articles

The Haber process, also called the Haber–Bosch process, is the main industrial procedure for the production of ammonia. The German chemists Fritz Haber and Carl Bosch developed it in the first decade of the 20th century. The process converts atmospheric nitrogen (N2) to ammonia (NH3) by a reaction with hydrogen (H2) using an iron metal catalyst under high temperatures and pressures. This reaction is slightly exothermic (i.e. it releases energy), meaning that the reaction is favoured at lower temperatures and higher pressures. It decreases entropy, complicating the process. Hydrogen is produced via steam reforming, followed by an iterative closed cycle to react hydrogen with nitrogen to produce ammonia.

Coke is a grey, hard, and porous coal-based fuel with a high carbon content and few impurities, made by heating coal or oil in the absence of air—a destructive distillation process. It is an important industrial product, used mainly in iron ore smelting, but also as a fuel in stoves and forges when air pollution is a concern.

Producer gas is fuel gas that is manufactured by blowing through a coke or coal fire with air and steam simultaneously. It mainly consists of carbon monoxide (CO), hydrogen (H2), as well as substantial amounts of nitrogen (N2). The caloric value of the producer gas is low (mainly because of its high nitrogen content), and the technology is obsolete. Improvements over producer gas, also obsolete, include water gas where the solid fuel is treated intermittently with air and steam and, far more efficiently synthesis gas where the solid fuel is replaced with methane.
Syngas, or synthesis gas, is a mixture of hydrogen and carbon monoxide, in various ratios. The gas often contains some carbon dioxide and methane. It is principally used for producing ammonia or methanol. Syngas is combustible and can be used as a fuel. Historically, it has been used as a replacement for gasoline, when gasoline supply has been limited; for example, wood gas was used to power cars in Europe during WWII.

The pyrolysis process is the thermal decomposition of materials at elevated temperatures, often in an inert atmosphere. The word is coined from the Greek-derived elements pyro "fire", "heat", "fever" and lysis "separating".

Gasification is a process that converts biomass- or fossil fuel-based carbonaceous materials into gases, including as the largest fractions: nitrogen (N2), carbon monoxide (CO), hydrogen (H2), and carbon dioxide (CO2). This is achieved by reacting the feedstock material at high temperatures (typically >700 °C), without combustion, via controlling the amount of oxygen and/or steam present in the reaction. The resulting gas mixture is called syngas (from synthesis gas) or producer gas and is itself a fuel due to the flammability of the H2 and CO of which the gas is largely composed. Power can be derived from the subsequent combustion of the resultant gas, and is considered to be a source of renewable energy if the gasified compounds were obtained from biomass feedstock.
Coal gas is a flammable gaseous fuel made from coal and supplied to the user via a piped distribution system. It is produced when coal is heated strongly in the absence of air. Town gas is a more general term referring to manufactured gaseous fuels produced for sale to consumers and municipalities.
The Fischer–Tropsch process (FT) is a collection of chemical reactions that converts a mixture of carbon monoxide and hydrogen, known as syngas, into liquid hydrocarbons. These reactions occur in the presence of metal catalysts, typically at temperatures of 150–300 °C (302–572 °F) and pressures of one to several tens of atmospheres. The Fischer–Tropsch process is an important reaction in both coal liquefaction and gas to liquids technology for producing liquid hydrocarbons.
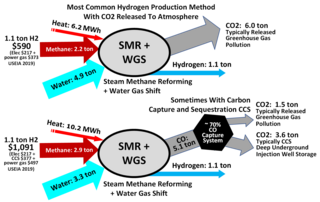
Steam reforming or steam methane reforming (SMR) is a method for producing syngas (hydrogen and carbon monoxide) by reaction of hydrocarbons with water. Commonly natural gas is the feedstock. The main purpose of this technology is hydrogen production. The reaction is represented by this equilibrium:
In industrial chemistry, coal gasification is the process of producing syngas—a mixture consisting primarily of carbon monoxide (CO), hydrogen, carbon dioxide, methane, and water vapour —from coal and water, air and/or oxygen.

The Sabatier reaction or Sabatier process produces methane and water from a reaction of hydrogen with carbon dioxide at elevated temperatures and pressures in the presence of a nickel catalyst. It was discovered by the French chemists Paul Sabatier and Jean-Baptiste Senderens in 1897. Optionally, ruthenium on alumina makes a more efficient catalyst. It is described by the following exothermic reaction:

Fuel gas is one of a number of fuels that under ordinary conditions are gaseous. Most fuel gases are composed of hydrocarbons, hydrogen, carbon monoxide, or mixtures thereof. Such gases are sources of energy that can be readily transmitted and distributed through pipes.
Coal liquefaction is a process of converting coal into liquid hydrocarbons: liquid fuels and petrochemicals. This process is often known as "Coal to X" or "Carbon to X", where X can be many different hydrocarbon-based products. However, the most common process chain is "Coal to Liquid Fuels" (CTL).

A wood gas generator is a gasification unit which converts timber or charcoal into wood gas, a producer gas consisting of atmospheric nitrogen, carbon monoxide, hydrogen, traces of methane, and other gases, which – after cooling and filtering – can then be used to power an internal combustion engine or for other purposes. Historically wood gas generators were often mounted on vehicles, but present studies and developments concentrate mostly on stationary plants.
The Dakota Gasification Company is a synthetic natural gas producing company founded in 1984 in Beulah, North Dakota, United States. It is an operator of the Great Plains Synfuels Plant. The plant is located at 47°21′27.75″N101°50′28.72″W. The plant uses lignite coal to produce synthetic natural gas utilizing a coal gasification process. The plant processes 16 thousand tons of coal daily. Coal is oxidized to coal gas, which is then converted from a mixture of carbon monoxide, carbon dioxide and hydrogen to methane, by hydrogenation over a nickel catalyst. The synthetic natural gas is pipelined to the Northern Border Pipeline which transports gas from Canada, Montana and North Dakota to the Ventura, Iowa area, where the pipeline interconnects with many pipelines supplying the eastern United States. The Dakota Gasification Company is a subsidiary of the Basin Electric Power Cooperative which is located in Bismarck, North Dakota. On August 16, 2021, it was announced Bakken Energy would be acquiring the Dakota Gasification Company to be transformed to a blue hydrogen project.
Water gas is a kind of fuel gas, a mixture of carbon monoxide and hydrogen. It is produced by "alternately hot blowing a fuel layer [coke] with air and gasifying it with steam". The caloric yield of this is about 10% of a modern syngas plant. Further making this technology unattractive, its precursor coke is expensive, whereas syngas uses cheaper precursor, mainly methane from natural gas.
Hydrogen production is the family of industrial methods for generating hydrogen gas. There are four main sources for the commercial production of hydrogen: natural gas, oil, coal, and electrolysis of water; which account for 48%, 30%, 18% and 4% of the world's hydrogen production respectively. Fossil fuels are the dominant source of industrial hydrogen. As of 2020, the majority of hydrogen (~95%) is produced by steam reforming of natural gas and other light hydrocarbons, partial oxidation of heavier hydrocarbons, and coal gasification. Other methods of hydrogen production include biomass gasification and methane pyrolysis. Methane pyrolysis and water electrolysis can use any source of electricity including renewable energy.
Rectisol is the trade name for an acid gas removal process that uses methanol as a solvent to separate acid gases such as hydrogen sulfide and carbon dioxide from valuable feed gas streams. By doing so, the feed gas is made more suitable for combustion and/or further processing. Rectisol is used most often to treat synthesis gas (primarily hydrogen and carbon monoxide) produced by gasification of coal or heavy hydrocarbons, as the methanol solvent is well able to remove trace contaminants such as ammonia, mercury, and hydrogen cyanide usually found in these gases. As an acid gas and large component of valuable feed gas streams, CO2 is separated during the methanol solvent regeneration.

The history of gaseous fuel, important for lighting, heating, and cooking purposes throughout most of the 19th century and the first half of the 20th century, began with the development of analytical and pneumatic chemistry in the 18th century. The manufacturing process for "synthetic fuel gases" typically consisted of the gasification of combustible materials, usually coal, but also wood and oil. The coal was gasified by heating the coal in enclosed ovens with an oxygen-poor atmosphere. The fuel gases generated were mixtures of many chemical substances, including hydrogen, methane, carbon monoxide and ethylene, and could be burnt for heating and lighting purposes. Coal gas, for example, also contains significant quantities of unwanted sulfur and ammonia compounds, as well as heavy hydrocarbons, and so the manufactured fuel gases needed to be purified before they could be used.
Lower-temperature fuel cell types such as the proton exchange membrane fuel cell, phosphoric acid fuel cell, and alkaline fuel cell require pure hydrogen as fuel, typically produced from external reforming of natural gas. However, fuels cells operating at high temperature such as the solid oxide fuel cell (SOFC) are not poisoned by carbon monoxide and carbon dioxide, and in fact can accept hydrogen, carbon monoxide, carbon dioxide, steam, and methane mixtures as fuel directly, because of their internal shift and reforming capabilities. This opens up the possibility of efficient fuel cell-based power cycles consuming solid fuels such as coal and biomass, the gasification of which results in syngas containing mostly hydrogen, carbon monoxide and methane which can be cleaned and fed directly to the SOFCs without the added cost and complexity of methane reforming, water gas shifting and hydrogen separation operations which would otherwise be needed to isolate pure hydrogen as fuel. A power cycle based on gasification of solid fuel and SOFCs is called an Integrated Gasification Fuel Cell (IGFC) cycle; the IGFC power plant is analogous to an integrated gasification combined cycle power plant, but with the gas turbine power generation unit replaced with a fuel cell power generation unit. By taking advantage of intrinsically high energy efficiency of SOFCs and process integration, exceptionally high power plant efficiencies are possible. Furthermore, SOFCs in the IGFC cycle can be operated so as to isolate a carbon dioxide-rich anodic exhaust stream, allowing efficient carbon capture to address greenhouse gas emissions concerns of coal-based power generation.
References
- 1 2 "Ludwig Mond". Web. HowStuffWorks. July 2010. Retrieved 17 Oct 2012.
- 1 2 3 Mond Gas. R.D. Wood & Co. 1903. p. 96 . Retrieved 14 Nov 2012.
- 1 2 3 4 5 Boak, Ken. "Gasification Historical archive - Mond Gas". Archived from the original on April 15, 2013. Retrieved October 17, 2012.
- 1 2 3 4 5 Thomas, Russell. "Producer Gas Plants". Web. academia.edu. Retrieved 17 Oct 2012.
- ↑ "Bures, Gas Works". Web. Retrieved 17 Oct 2012.
