
A Bunsen burner, named after Robert Bunsen, is a kind of ambient air gas burner used as laboratory equipment; it produces a single open gas flame, and is used for heating, sterilization, and combustion.

Combustion, or burning, is a high-temperature exothermic redox chemical reaction between a fuel and an oxidant, usually atmospheric oxygen, that produces oxidized, often gaseous products, in a mixture termed as smoke. Combustion does not always result in fire, because a flame is only visible when substances undergoing combustion vaporize, but when it does, a flame is a characteristic indicator of the reaction. While activation energy must be supplied to initiate combustion, the heat from a flame may provide enough energy to make the reaction self-sustaining.

Fire is the rapid oxidation of a material in the exothermic chemical process of combustion, releasing heat, light, and various reaction products. At a certain point in the combustion reaction, called the ignition point, flames are produced. The flame is the visible portion of the fire. Flames consist primarily of carbon dioxide, water vapor, oxygen and nitrogen. If hot enough, the gases may become ionized to produce plasma. Depending on the substances alight, and any impurities outside, the color of the flame and the fire's intensity will be different.

Propane is a three-carbon alkane with the molecular formula C3H8. It is a gas at standard temperature and pressure, but compressible to a transportable liquid. A by-product of natural gas processing and petroleum refining, it is commonly used as a fuel in domestic and industrial applications and in low-emissions public transportation. Discovered in 1857 by the French chemist Marcellin Berthelot, it became commercially available in the US by 1911. Propane is one of a group of liquefied petroleum gases. The others include propylene, butane, butylene, butadiene, isobutylene, and mixtures thereof. Propane has lower volumetric energy density, but higher gravimetric energy density and burns more cleanly than gasoline and coal.

A flame is the visible, gaseous part of a fire. It is caused by a highly exothermic chemical reaction taking place in a thin zone. When flames are hot enough to have ionized gaseous components of sufficient density they are then considered plasma.

The fire triangle or combustion triangle is a simple model for understanding the necessary ingredients for most fires.

MAPP gas was a trademarked name, belonging to The Linde Group, a division of the former global chemical giant Union Carbide, for a fuel gas based on a stabilized mixture of methylacetylene (propyne), propadiene and propane. The name comes from the original chemical composition, methylacetylene-propadiene propane. "MAPP gas" is also widely used as a generic name for UN 1060 stabilised methylacetylene-propadiene.

Oxyhydrogen is a mixture of hydrogen (H2) and oxygen (O2) gases. This gaseous mixture is used for torches to process refractory materials and was the first gaseous mixture used for welding. Theoretically, a ratio of 2:1 hydrogen:oxygen is enough to achieve maximum efficiency; in practice a ratio 4:1 or 5:1 is needed to avoid an oxidizing flame.

In the study of combustion, the adiabatic flame temperature is the temperature reached by a flame under ideal conditions. It is an upper bound of the temperature that is reached in actual processes.

A kerosene heater, also known as a paraffin heater, is typically a portable, unvented, kerosene-fueled, space heating device. In Japan and other countries, they are a primary source of home heat. In the United States and Australia, they are a supplemental heat or a source of emergency heat during a power outage. Most kerosene heaters produce between 3.3 and 6.8 kilowatts.

A LO NOx burner is a type of burner that is typically used in utility boilers to produce steam and electricity.

A propane torch is a tool normally used for the application of flame or heat which uses propane, a hydrocarbon gas, for its fuel and ambient air as its combustion medium. Propane is one of a group of by-products of the natural gas and petroleum industries known as liquefied petroleum gas (LPG). Propane and other fuel torches are most commonly used in the manufacturing, construction and metal-working industries.

Thermal spraying techniques are coating processes in which melted materials are sprayed onto a surface. The "feedstock" is heated by electrical or chemical means.
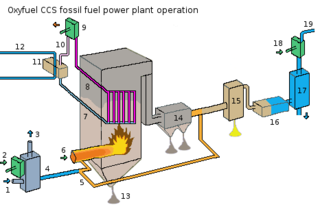
Oxy-fuel combustion is the process of burning a fuel using pure oxygen, or a mixture of oxygen and recirculated flue gas, instead of air. Since the nitrogen component of air is not heated, fuel consumption is reduced, and higher flame temperatures are possible. Historically, the primary use of oxy-fuel combustion has been in welding and cutting of metals, especially steel, since oxy-fuel allows for higher flame temperatures than can be achieved with an air-fuel flame. It has also received a lot of attention in recent decades as a potential carbon capture and storage technology.

Oxy-fuel welding and oxy-fuel cutting are processes that use fuel gases and oxygen to weld or cut metals. French engineers Edmond Fouché and Charles Picard became the first to develop oxygen-acetylene welding in 1903. Pure oxygen, instead of air, is used to increase the flame temperature to allow localised melting of the workpiece material in a room environment. A common propane/air flame burns at about 2,250 K, a propane/oxygen flame burns at about 2,526 K, an oxyhydrogen flame burns at 3,073 K and an acetylene/oxygen flame burns at about 3,773 K.

A blowtorch, also referred to as a blowlamp, is an ambient air fuel-burning gas lamp used for applying flame and heat to various applications, usually metalworking.

A luminous flame is a burning flame which is brightly visible. Much of its output is in the form of visible light, as well as heat or light in the non-visible wavelengths.
Flame treatment is the application of a gas flame to the surface of a material to improve adhesion.

An industrial furnace, also known as a direct heater or a direct fired heater, is a device used to provide heat for an industrial process, typically higher than 400 degrees Celsius. They are used to provide heat for a process or can serve as reactor which provides heats of reaction. Furnace designs vary as to its function, heating duty, type of fuel and method of introducing combustion air. Heat is generated by an industrial furnace by mixing fuel with air or oxygen, or from electrical energy. The residual heat will exit the furnace as flue gas. These are designed as per international codes and standards the most common of which are ISO 13705 / American Petroleum Institute (API) Standard 560. Types of industrial furnaces include batch ovens, metallurgical furnaces, vacuum furnaces, and solar furnaces. Industrial furnaces are used in applications such as chemical reactions, cremation, oil refining, and glasswork.

Steam cracking is a petrochemical process in which saturated hydrocarbons are broken down into smaller, often unsaturated, hydrocarbons. It is the principal industrial method for producing the lighter alkenes, including ethene and propene. Steam cracker units are facilities in which a feedstock such as naphtha, liquefied petroleum gas (LPG), ethane, propane or butane is thermally cracked through the use of steam in steam cracking furnaces to produce lighter hydrocarbons. The propane dehydrogenation process may be accomplished through different commercial technologies. The main differences between each of them concerns the catalyst employed, design of the reactor and strategies to achieve higher conversion rates.























