Related Research Articles

Psychopharmacology is the scientific study of the effects drugs have on mood, sensation, thinking, behavior, judgment and evaluation, and memory. It is distinguished from neuropsychopharmacology, which emphasizes the correlation between drug-induced changes in the functioning of cells in the nervous system and changes in consciousness and behavior.

Stimulants are a class of drugs that increase the activity of the brain. They are used for various purposes, such as enhancing alertness, attention, motivation, cognition, mood, and physical performance. Some of the most common stimulants are caffeine, nicotine, amphetamines, cocaine, methylphenidate, and modafinil.
A dopamine reuptake inhibitor (DRI) is a class of drug which acts as a reuptake inhibitor of the monoamine neurotransmitter dopamine by blocking the action of the dopamine transporter (DAT). Reuptake inhibition is achieved when extracellular dopamine not absorbed by the postsynaptic neuron is blocked from re-entering the presynaptic neuron. This results in increased extracellular concentrations of dopamine and increase in dopaminergic neurotransmission.

Dopamine receptors are a class of G protein-coupled receptors that are prominent in the vertebrate central nervous system (CNS). Dopamine receptors activate different effectors through not only G-protein coupling, but also signaling through different protein interactions. The neurotransmitter dopamine is the primary endogenous ligand for dopamine receptors.
Substance dependence, also known as drug dependence, is a biopsychological situation whereby an individual's functionality is dependent on the necessitated re-consumption of a psychoactive substance because of an adaptive state that has developed within the individual from psychoactive substance consumption that results in the experience of withdrawal and that necessitates the re-consumption of the drug. A drug addiction, a distinct concept from substance dependence, is defined as compulsive, out-of-control drug use, despite negative consequences. An addictive drug is a drug which is both rewarding and reinforcing. ΔFosB, a gene transcription factor, is now known to be a critical component and common factor in the development of virtually all forms of behavioral and drug addictions, but not dependence.

The κ-opioid receptor or kappa opioid receptor, abbreviated KOR or KOP for its ligand ketazocine, is a G protein-coupled receptor that in humans is encoded by the OPRK1 gene. The KOR is coupled to the G protein Gi/G0 and is one of four related receptors that bind opioid-like compounds in the brain and are responsible for mediating the effects of these compounds. These effects include altering nociception, consciousness, motor control, and mood. Dysregulation of this receptor system has been implicated in alcohol and drug addiction.

George F. Koob is a Professor and former Chair of the Committee on the Neurobiology of Addictive Disorders at the Scripps Research Institute and Adjunct Professor of Psychology, Psychiatry, and Skaggs School of Pharmacy and Pharmaceutical Sciences at the University of California, San Diego. In 2014 he became the director of the National Institute on Alcohol Abuse and Alcoholism.

Cocaine- and amphetamine-regulated transcript, also known as CART, is a neuropeptide protein that in humans is encoded by the CARTPT gene. CART appears to have roles in reward, feeding, and stress, and it has the functional properties of an endogenous psychostimulant.

Phenyltropanes (PTs) were originally developed to reduce cocaine addiction and dependency. In general these compounds act as inhibitors of the plasmalemmal monoamine reuptake transporters. This research has spanned beyond the last couple decades, and has picked up its pace in recent times, creating numerous phenyltropanes as research into cocaine analogues garners interest to treat addiction.

Troparil is a stimulant drug used in scientific research. Troparil is a phenyltropane-based dopamine reuptake inhibitor (DRI) that is derived from methylecgonidine. Troparil is a few times more potent than cocaine as a dopamine reuptake inhibitor, but is less potent as a serotonin reuptake inhibitor, and has a duration spanning a few times longer, since the phenyl ring is directly connected to the tropane ring through a non-hydrolyzable carbon-carbon bond. The lack of an ester linkage removes the local anesthetic action from the drug, so troparil is a pure stimulant. This change in activity also makes troparil slightly less cardiotoxic than cocaine. The most commonly used form of troparil is the tartrate salt, but the hydrochloride and naphthalenedisulfonate salts are also available, as well as the free base.
Psychological dependence is a cognitive disorder that involves emotional–motivational withdrawal symptoms – such as anxiety or anhedonia – upon cessation of prolonged drug abuse or certain repetitive behaviors. It develops through frequent exposure to certain psychoactive substances or behaviors, which leads to an individual requiring further exposure to avoid withdrawal symptoms, as a result of negative reinforcement. Neuronal counter-adaptation is believed to play a role in generating withdrawal symptoms, which could be mediated through changes in neurotransmitter activity or altered receptor expression. Environmental enrichment and physical activity can attenuate withdrawal symptoms.

RTI(-4229)-150, is a phenyltropane derivative which acts as a potent dopamine reuptake inhibitor and stimulant drug. It is around 5x more potent than cocaine, but is more selective for the dopamine transporter relative to the other monoamine transporters. RTI-150 has a fast onset of effects and short duration of action, and its abuse potential in animal studies is similar to that of cocaine itself; its main application in scientific research has been in studies investigating the influence of pharmacokinetics on the abuse potential of stimulant drugs, with the rapid entry of RTI-150 into the brain thought to be a key factor in producing its high propensity for development of dependence in animals. RTI-150 is not explicitly illegal anywhere in the world, but its similar structure and pharmacological activity to cocaine makes it possible that it would be considered a controlled substance analogue in countries such as the US, Canada, Australia and New Zealand which have controlled substance analogue legislation.

RTI-126 is a phenyltropane derivative which acts as a potent monoamine reuptake inhibitor and stimulant drug, and has been sold as a designer drug. It is around 5 times more potent than cocaine at inhibiting monoamine reuptake in vitro, but is relatively unselective. It binds to all three monoamine transporters, although still with some selectivity for the dopamine transporter. RTI-126 has a fast onset of effects and short duration of action, and its pharmacological profile in animals is among the closest to cocaine itself out of all the drugs in the RTI series. Its main application in scientific research has been in studies investigating the influence of pharmacokinetics on the abuse potential of stimulant drugs, with its rapid entry into the brain thought to be a key factor in producing its high propensity for development of dependence in animals.
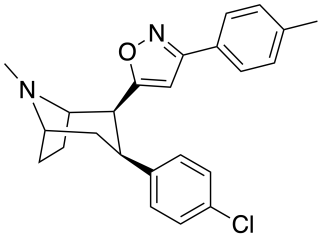
RTI(-4229)-336, is a phenyltropane derivative which acts as a potent and selective dopamine reuptake inhibitor and stimulant drug. It binds to the dopamine transporter with around 20x the affinity of cocaine, however it produces relatively mild stimulant effects, with a slow onset and long duration of action. These characteristics make it a potential candidate for treatment of cocaine addiction, as a possible substitute drug analogous to how methadone is used for treating heroin abuse. RTI-336 fully substitutes for cocaine in addicted monkeys and supports self-administration, and significantly reduces rates of cocaine use, especially when combined with SSRIs, and research is ongoing to determine whether it could be a viable substitute drug in human cocaine addicts.
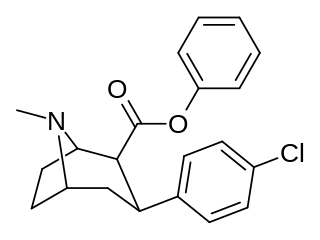
RTI(-4229)-113 is a stimulant drug which acts as a potent and fully selective dopamine reuptake inhibitor (DRI). It has been suggested as a possible substitute drug for the treatment of cocaine addiction. "RTI-113 has properties that make it an ideal medication for cocaine abusers, such as an equivalent efficacy, a higher potency, and a longer duration of action as compared to cocaine." Replacing the methyl ester in RTI-31 with a phenyl ester makes the resultant RTI-113 fully DAT specific. RTI-113 is a particularly relevant phenyltropane cocaine analog that has been tested on squirrel monkeys. RTI-113 has also been tested against cocaine in self-administration studies for DAT occupancy by PET on awake rhesus monkeys. The efficacy of cocaine analogs to elicit self-administration is closely related to the rate at which they are administered. Slower onset of action analogs are less likely to function as positive reinforcers than analogues that have a faster rate of onset.
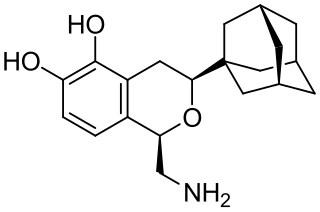
A-77636 is a synthetic drug which acts as a selective D1 receptor full agonist. It has nootropic, anorectic, rewarding and antiparkinsonian effects in animal studies, but its high potency and long duration of action causes D1 receptor downregulation and tachyphylaxis, and unlike other D1 full agonists such as SKF-82,958, it does not produce place preference in animals. A-77636 partially substituted for cocaine in animal studies, and has been suggested for use as a possible substitute drug in treating addiction, but it is better known for its use in studying the role of D1 receptors in the brain.
Addiction is a state characterized by compulsive engagement in rewarding stimuli, despite adverse consequences. The process of developing an addiction occurs through instrumental learning, which is otherwise known as operant conditioning.
Addiction vulnerability is an individual's risk of developing an addiction during their lifetime. There are a range of genetic and environmental risk factors for developing an addiction that vary across the population. Genetic and environmental risk factors each account for roughly half of an individual's risk for developing an addiction; the contribution from epigenetic risk factors to the total risk is unknown. Even in individuals with a relatively low genetic risk, exposure to sufficiently high doses of an addictive drug for a long period of time can result in an addiction. In other words, anyone can become an individual with a substance use disorder under particular circumstances. Research is working toward establishing a comprehensive picture of the neurobiology of addiction vulnerability, including all factors at work in propensity for addiction.

Patricia Janak is a Bloomberg Distinguished Professor at Johns Hopkins University who studies the biological basis of behavior through associative learning. Janak applies this research to pathological behaviors, such as addiction and posttraumatic stress disorder, to improve understanding of how stimuli affect relapse and responses.
Nancy Rutledge Zahniser was an American pharmacologist, best known for her work involving the mechanism of dopaminergic pathways and chemical modifications of them. Although born in Ann Arbor, Michigan, Zahniser grew up in Chillicothe, Ohio and subsequently enrolled at the College of Wooster, where she obtained a degree in chemistry. After completing her degree, Zahniser spent some time in India where she met her first husband Mark Zahniser; she later returned to the United States to attend the University of Pittsburgh School of Pharmacy, where she earned her PhD in pharmacology in 1977. Zahniser went on to complete her post-doctoral training at the University of Colorado Health Sciences Center's Department of Pharmacology and then became a part of the faculty there. In 2007, she became associate dean for research education. She played a role in advancing the careers of many post-doctoral students in her lab. In addition to her work as a professor, Zahniser was also a member of several boards, committees, review panels, and professional societies related to pharmacology, neuroscience, and addiction. She led several national research meetings from 1995-2002.
References
- ↑ "Doctors of Philosophy in the Faculty of Arts and Sciences". Conferring of Degrees at the close of the ninety-fourth academic year (PDF). Baltimore, Maryland: The Johns Hopkins University. May 27, 1970. p. 52. Retrieved November 4, 2020.
- ↑ "Google".
- ↑ "Archived copy" (PDF). Archived from the original (PDF) on 2014-08-26. Retrieved 2014-08-24.
{{cite web}}: CS1 maint: archived copy as title (link) - ↑ "The Laboratory of Dr. Michael J. Kuhar". Emory University. Retrieved 3 June 2014.
- ↑ "Conversation with Michael Kuhar". Addiction. 105 (4): 593–600. 2010. doi:10.1111/j.1360-0443.2010.02897.x. ISSN 0965-2140.
- ↑ Campbell, Nancy D. (2007). ""The Hijacked Brain": Reimagining Addiction". Discovering Addiction: The Science and Politics of Substance Abuse Research. University of Michigan Press. p. 200–221. ISBN 978-0-472-11610-2. JSTOR j.ctvnjbdtz.15 . Retrieved 2024-07-16.
- ↑ Okuda, Takashi; Haga, Tatsuya; Kanai, Yoshikatsu; Endou, Hitoshi; Ishihara, Takeshi; Katsura, Isao (2000). "Identification and characterization of the high-affinity choline transporter". Nature Neuroscience. 3 (2). Springer Science and Business Media LLC: 120–125. doi:10.1038/72059. ISSN 1097-6256.
- ↑ Iversen, Leslie; Iversen, Susan; Bloom, Floyd E.; Roth, Robert H. (2009). Introduction to Neuropsychopharmacology. New York: Oxford University Press. p. 130. ISBN 0-19-538053-3.
- ↑ Frey, Kirk A.; Albin, Roger L. (1997). "Receptor Binding Techniques". Current Protocols in Neuroscience. 00 (1). doi:10.1002/0471142301.ns0104s00. ISSN 1934-8584.
- ↑ Snyder, Solomon H. (1996). Drugs and the Brain. New York, NY: Times Books. p. 43. ISBN 0-7167-6017-7.
- ↑ Push, S (1984) "PET scans are now being used to study the distribution of dopamine receptors inpatients with schizophrenia and other psychiatric and neurologic disorders". Hopkins Medical News 8(12).
- ↑ Sehlstedt, Al (1983) "Scientists observe living brain’s receptors". The Sun (Baltimore, MD) Tuesday, September 20.
- ↑ Pierce, R. Christopher; Kumaresan, Vidhya (2006). "The mesolimbic dopamine system: The final common pathway for the reinforcing effect of drugs of abuse?". Neuroscience & Biobehavioral Reviews. 30 (2). Elsevier BV: 215–238. doi:10.1016/j.neubiorev.2005.04.016. ISSN 0149-7634.
- ↑ "Cocaine Addition Linked To Brain Protein". AP NEWS. Retrieved 2023-06-21.
- 1 2 Runyon, Scott; Carroll, F. Ivy (2006-09-01). "Dopamine Transporter Ligands: Recent Developments and Therapeutic Potential". Current Topics in Medicinal Chemistry. 6 (17). Bentham Science Publishers Ltd.: 1825–1843. doi:10.2174/156802606778249775. ISSN 1568-0266.
- ↑ http://www.Yerkes.emory.edu/research/divions/behavioral_neuroscience/kuhar_michael.html [ permanent dead link ]
- ↑ Parvin, PP. Michael Kuhar on Getting Collegial, in Emory magazine Spring 2014, p 16.
- ↑ "IDARS NEWS" (PDF). Archived from the original (PDF) on February 23, 2017.
- ↑ "The College on Problems of Drug Dependence". www.cpdd.vcu.edu. Archived from the original on 2005-12-20.
- ↑ "Efron Award Past Winners - ACNP". Archived from the original on 2015-03-08. Retrieved 2014-06-11.
- ↑ "ASPET | ASPET Award Winners".
- ↑ "The College on Problems of Drug Dependence". www.cpdd.vcu.edu. Archived from the original on 2005-12-04.