Micro process engineering is the science of conducting chemical or physical processes (unit operations) inside small volumina, typically inside channels with diameters of less than 1 mm (microchannels) or other structures with sub-millimeter dimensions. These processes are usually carried out in continuous flow mode, as opposed to batch production, allowing a throughput high enough to make micro process engineering a tool for chemical production. Micro process engineering is therefore not to be confused with microchemistry, which deals with very small overall quantities of matter.

In chemical engineering and related fields, a unit operation is a basic step in a process. Unit operations involve a physical change or chemical transformation such as separation, crystallization, evaporation, filtration, polymerization, isomerization, and other reactions. For example, in milk processing, homogenization, pasteurization, and packaging are each unit operations which are connected to create the overall process. A process may require many unit operations to obtain the desired product from the starting materials, or feedstocks.

Batch production is a technique used in manufacturing, in which the object in question is created stage by stage over a series of workstations, and different batches of products are made. Together with job production and mass production it is one of the three main production methods.
The subfield of micro process engineering that deals with chemical reactions, carried out in microstructured reactors or "microreactors", is also known as microreaction technology.
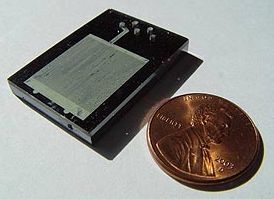
A microreactor or microstructured reactor or microchannel reactor is a device in which chemical reactions take place in a confinement with typical lateral dimensions below 1 mm; the most typical form of such confinement are microchannels. Microreactors are studied in the field of micro process engineering, together with other devices in which physical processes occur. The microreactor is usually a continuous flow reactor. Microreactors offer many advantages over conventional scale reactors, including vast improvements in energy efficiency, reaction speed and yield, safety, reliability, scalability, on-site/on-demand production, and a much finer degree of process control.
The unique advantages of microstructured reactors or microreactors are enhanced heat transfer due to the large surface area-to-volume ratio, and enhanced mass transfer. For example, the length scale of diffusion processes is comparable to that of microchannels or even shorter, and efficient mixing of reactants can be achieved during very short times (typically milliseconds). The good heat transfer properties allow a precise temperature control of reactions. For example, highly exothermic reactions can be conducted almost isothermally when the microstructured reactor contains a second set of microchannels ("cooling passage"), fluidically separated from the reaction channels ("reaction passage"), through which a flow of cold fluid with sufficiently high heat capacity is maintained. It is also possible to change the temperature of microstructured reactors very rapidly to intentionally achieve a non-isothermal behaviour.
Heat exchangers were initially developed to use plain heat transfer surfaces. An Enhanced heat transfer surface has a special surface geometry that provides a higher thermal performance, per unit base surface area than a plain surface.
Mass transfer is the net movement of mass from one location, usually meaning stream, phase, fraction or component, to another. Mass transfer occurs in many processes, such as absorption, evaporation, drying, precipitation, membrane filtration, and distillation. Mass transfer is used by different scientific disciplines for different processes and mechanisms. The phrase is commonly used in engineering for physical processes that involve diffusive and convective transport of chemical species within physical systems.

Diffusion is the net movement of molecules or atoms from a region of higher concentration to a region of lower concentration. Diffusion is driven by a gradient in chemical potential of the diffusing species.

A bioreactor may refers to any manufactured device or system that supports a biologically active environment. In one case, a bioreactor is a vessel in which a chemical process is carried out which involves organisms or biochemically active substances derived from such organisms. This process can either be aerobic or anaerobic. These bioreactors are commonly cylindrical, ranging in size from litres to cubic metres, and are often made of stainless steel.

Fluidization is a process similar to liquefaction whereby a granular material is converted from a static solid-like state to a dynamic fluid-like state. This process occurs when a fluid is passed up through the granular material.
A chemical reactor is an enclosed volume in which a chemical reaction takes place. In chemical engineering, it is generally understood to be a process vessel used to carry out a chemical reaction, which is one of the classic unit operations in chemical process analysis. The design of a chemical reactor deals with multiple aspects of chemical engineering. Chemical engineers design reactors to maximize net present value for the given reaction. Designers ensure that the reaction proceeds with the highest efficiency towards the desired output product, producing the highest yield of product while requiring the least amount of money to purchase and operate. Normal operating expenses include energy input, energy removal, raw material costs, labor, etc. Energy changes can come in the form of heating or cooling, pumping to increase pressure, frictional pressure loss or agitation.
Thermofluids is a branch of science and engineering encompassing four intersecting fields:
Microwave chemistry is the science of applying microwave radiation to chemical reactions. Microwaves act as high frequency electric fields and will generally heat any material containing mobile electric charges, such as polar molecules in a solvent or conducting ions in a solid. Polar solvents are heated as their component molecules are forced to rotate with the field and lose energy in collisions. Semiconducting and conducting samples heat when ions or electrons within them form an electric current and energy is lost due to the electrical resistance of the material. Microwave heating in the laboratory began to gain wide acceptance following papers in 1986, although the use of microwave heating in chemical modification can be traced back to the 1950s. Although occasionally known by such acronyms as MAOS, MEC or MORE synthesis, these acronyms have had little acceptance outside a small number of groups.
The continuous stirred-tank reactor (CSTR), also known as vat- or backmix reactor, or a continuous-flow stirred-tank reactor (CFSTR), is a common model for a chemical reactor in chemical engineering. A CSTR often refers to a model used to estimate the key unit operation variables when using a continuous agitated-tank reactor to reach a specified output. The mathematical model works for all fluids: liquids, gases, and slurries.

The plug flow reactor model is a model used to describe chemical reactions in continuous, flowing systems of cylindrical geometry. The PFR model is used to predict the behavior of chemical reactors of such design, so that key reactor variables, such as the dimensions of the reactor, can be estimated.
The batch reactor is the generic term for a type of vessel widely used in the process industries. Its name is something of a misnomer since vessels of this type are used for a variety of process operations such as solids dissolution, product mixing, chemical reactions, batch distillation, crystallization, liquid/liquid extraction and polymerization. In some cases, they are not referred to as reactors but have a name which reflects the role they perform.
In flow chemistry, a chemical reaction is run in a continuously flowing stream rather than in batch production. In other words, pumps move fluid into a tube, and where tubes join one another, the fluids contact one another. If these fluids are reactive, a reaction takes place. Flow chemistry is a well-established technique for use at a large scale when manufacturing large quantities of a given material. However, the term has only been coined recently for its application on a laboratory scale. Often, microreactors are used.

A reaction calorimeter is a calorimeter that measures the amount of energy released (exothermic) or absorbed (endothermic) by a chemical reaction. These measurements provide a more accurate picture of such reactions.
Micro heat exchangers, Micro-scale heat exchangers, or microstructured heat exchangers are heat exchangers in which fluid flows in lateral confinements with typical dimensions below 1 mm. The most typical such confinement are microchannels, which are channels with a hydraulic diameter below 1 mm. Microchannel heat exchangers can be made from metal, ceramic,

The Institute for Micro Process Engineering IMVT is an institute within the Karlsruhe Research Center in Eggenstein-Leopoldshafen, Germany. Its main field of activity is micro process engineering, the science of conducting chemical and/or physical processes in confines with typical dimensions below 1 mm.

A fluidized bed reactor (FBR) is a type of reactor device that can be used to carry out a variety of multiphase chemical reactions. In this type of reactor, a fluid is passed through a solid granular material at high enough velocities to suspend the solid and cause it to behave as though it were a fluid. This process, known as fluidization, imparts many important advantages to the FBR. As a result, the fluidized bed reactor is now used in many industrial applications.
Chemical vapour infiltration (CVI) is a ceramic engineering process whereby matrix material is infiltrated into fibrous preforms by the use of reactive gases at elevated temperature to form fiber-reinforced composites. The earliest use of CVI was the infiltration of fibrous alumina with chromium carbide. CVI can be applied to the production of carbon-carbon composites and ceramic matrix composites. A similar technique is chemical vapour deposition (CVD), the main difference being that the deposition process of CVD is on hot bulk surfaces, while the deposition process of CVI is on porous substrates.
Microchannel in microtechnology is a channel with a hydraulic diameter below 1 mm. Microchannels are used in fluid control and heat transfer. The concept of the microchannel was proposed for the first time by researchers Tuckerman and Pease of Stanford Electronics Laboratories. They suggested an effective method for designing microchannels in the laminar and fully developed flow.
Continuous reactors carry material as a flowing stream. Reactants are continuously fed into the reactor and emerge as continuous stream of product. Continuous reactors are used for a wide variety of chemical and biological processes within the food, chemical and pharmaceutical industries. A survey of the continuous reactor market will throw up a daunting variety of shapes and types of machine. Beneath this variation however lies a relatively small number of key design features which determine the capabilities of the reactor. When classifying continuous reactors, it can be more helpful to look at these design features rather than the whole system.

In chemical engineering, a jacketed vessel is a container that is designed for controlling temperature of its contents, by using a cooling or heating "jacket" around the vessel through which a cooling or heating fluid is circulated.
Micro-combustion is the sequence of exothermic chemical reaction between a fuel and an oxidant accompanied by the production of heat and conversion of chemical species at micro level. The release of heat can result in the production of light in the form of either glowing or a flame. Fuels of interest often include organic compounds in the gas, liquid or solid phase. The major problem of micro-combustion is the high surface to volume ratio. As the surface to volume ratio increases heat loss to walls of combustor increases which leads to flame quenching.