
Chemical engineering is an engineering field which deals with the study of operation and design of chemical plants as well as methods of improving production. Chemical engineers develop economical commercial processes to convert raw materials into useful products. Chemical engineering uses principles of chemistry, physics, mathematics, biology, and economics to efficiently use, produce, design, transport and transform energy and materials. The work of chemical engineers can range from the utilization of nanotechnology and nanomaterials in the laboratory to large-scale industrial processes that convert chemicals, raw materials, living cells, microorganisms, and energy into useful forms and products. Chemical engineers are involved in many aspects of plant design and operation, including safety and hazard assessments, process design and analysis, modeling, control engineering, chemical reaction engineering, nuclear engineering, biological engineering, construction specification, and operating instructions.
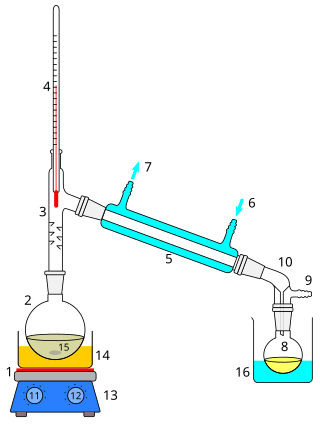
Distillation, also classical distillation, is the process of separating the component substances of a liquid mixture of two or more chemically discrete substances; the separation process is realized by way of the selective boiling of the mixture and the condensation of the vapors in a still.
Mass transfer is the net movement of mass from one location to another. Mass transfer occurs in many processes, such as absorption, evaporation, drying, precipitation, membrane filtration, and distillation. Mass transfer is used by different scientific disciplines for different processes and mechanisms. The phrase is commonly used in engineering for physical processes that involve diffusive and convective transport of chemical species within physical systems.

A fractionating column or fractional column is equipment used in the distillation of liquid mixtures to separate the mixture into its component parts, or fractions, based on their differences in volatility. Fractionating columns are used in small-scale laboratory distillations as well as large-scale industrial distillations.

Crystallization is the process by which solids form, where the atoms or molecules are highly organized into a structure known as a crystal. Some ways by which crystals form are precipitating from a solution, freezing, or more rarely deposition directly from a gas. Attributes of the resulting crystal depend largely on factors such as temperature, air pressure, cooling rate, and in the case of liquid crystals, time of fluid evaporation.

Continuous distillation, a form of distillation, is an ongoing separation in which a mixture is continuously fed into the process and separated fractions are removed continuously as output streams. Distillation is the separation or partial separation of a liquid feed mixture into components or fractions by selective boiling and condensation. The process produces at least two output fractions. These fractions include at least one volatile distillate fraction, which has boiled and been separately captured as a vapor condensed to a liquid, and practically always a bottoms fraction, which is the least volatile residue that has not been separately captured as a condensed vapor.

In chemical processing, a packed bed is a hollow tube, pipe, or other vessel that is filled with a packing material. The packed bed can be randomly filled with small objects like Raschig rings or else it can be a specifically designed structured packing. Packed beds may also contain catalyst particles or adsorbents such as zeolite pellets, granular activated carbon, etc.
In a scientific sense, a chemical process is a method or means of somehow changing one or more chemicals or chemical compounds. Such a chemical process can occur by itself or be caused by an outside force, and involves a chemical reaction of some sort. In an "engineering" sense, a chemical process is a method intended to be used in manufacturing or on an industrial scale to change the composition of chemical(s) or material(s), usually using technology similar or related to that used in chemical plants or the chemical industry.
This is an alphabetical list of articles pertaining specifically to chemical engineering.
A theoretical plate in many separation processes is a hypothetical zone or stage in which two phases, such as the liquid and vapor phases of a substance, establish an equilibrium with each other. Such equilibrium stages may also be referred to as an equilibrium stage, ideal stage, or a theoretical tray. The performance of many separation processes depends on having series of equilibrium stages and is enhanced by providing more such stages. In other words, having more theoretical plates increases the efficiency of the separation process be it either a distillation, absorption, chromatographic, adsorption or similar process.

An evaporator is a type of heat exchanger device that facilitates evaporation by utilizing conductive and convective heat transfer, which provides the necessary thermal energy for phase transition from liquid to vapor. Within evaporators, a circulating liquid is exposed to an atmospheric or reduced pressure environment, causing it to boil at a lower temperature compared to normal atmospheric boiling.
The following outline is provided as an overview of and topical guide to chemical engineering:
Stripping is a physical separation process where one or more components are removed from a liquid stream by a vapor stream. In industrial applications the liquid and vapor streams can have co-current or countercurrent flows. Stripping is usually carried out in either a packed or trayed column.
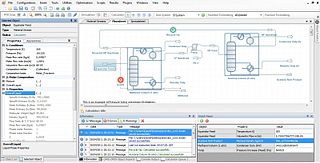
Process simulation is used for the design, development, analysis, and optimization of technical process of simulation of processes such as: chemical plant s, chemical processes, environmental systems, power stations, complex manufacturing operations, biological processes, and similar technical functions.
In engineering, physics, and chemistry, the study of transport phenomena concerns the exchange of mass, energy, charge, momentum and angular momentum between observed and studied systems. While it draws from fields as diverse as continuum mechanics and thermodynamics, it places a heavy emphasis on the commonalities between the topics covered. Mass, momentum, and heat transport all share a very similar mathematical framework, and the parallels between them are exploited in the study of transport phenomena to draw deep mathematical connections that often provide very useful tools in the analysis of one field that are directly derived from the others.
Membrane technology encompasses the scientific processes used in the construction and application of membranes. Membranes are used to facilitate the transport or rejection of substances between mediums, and the mechanical separation of gas and liquid streams. In the simplest case, filtration is achieved when the pores of the membrane are smaller than the diameter of the undesired substance, such as a harmful microorganism. Membrane technology is commonly used in industries such as water treatment, chemical and metal processing, pharmaceuticals, biotechnology, the food industry, as well as the removal of environmental pollutants.
A separation process is a method that converts a mixture or a solution of chemical substances into two or more distinct product mixtures, a scientific process of separating two or more substances in order to obtain purity. At least one product mixture from the separation is enriched in one or more of the source mixture's constituents. In some cases, a separation may fully divide the mixture into pure constituents. Separations exploit differences in chemical properties or physical properties between the constituents of a mixture.
Industrial separation processes are technical procedures which are used in industry to separate a product from impurities or other products. The original mixture may either be a natural resource or the product of a chemical reaction.
Aspen Plus, Aspen HYSYS, ChemCad and MATLAB, PRO are the commonly used process simulators for modeling, simulation and optimization of a distillation process in the chemical industries. Distillation is the technique of preferential separation of the more volatile components from the less volatile ones in a feed followed by condensation. The vapor produced is richer in the more volatile components. The distribution of the component in the two phase is governed by the vapour-liquid equilibrium relationship. In practice, distillation may be carried out by either two principal methods. The first method is based on the production of vapor boiling the liquid mixture to be separated and condensing the vapors without allowing any liquid to return to the still. There is no reflux. The second method is based on the return of part of the condensate to still under such conditions that this returning liquid is brought into intimate contact with the vapors on their way to condenser.








